Anat Cell Biol.
2020 Dec;53(4):460-470. 10.5115/acb.20.193.
Effect of cypermethrin on the postnatal development of the medulla oblongata and the possible protective role of melatonin in albino rats
- Affiliations
-
- 1Department of Anatomy and Embryology, Faculty of Medicine, Menoufia University, Shebin El‐Kom, Egypt
- KMID: 2509692
- DOI: http://doi.org/10.5115/acb.20.193
Abstract
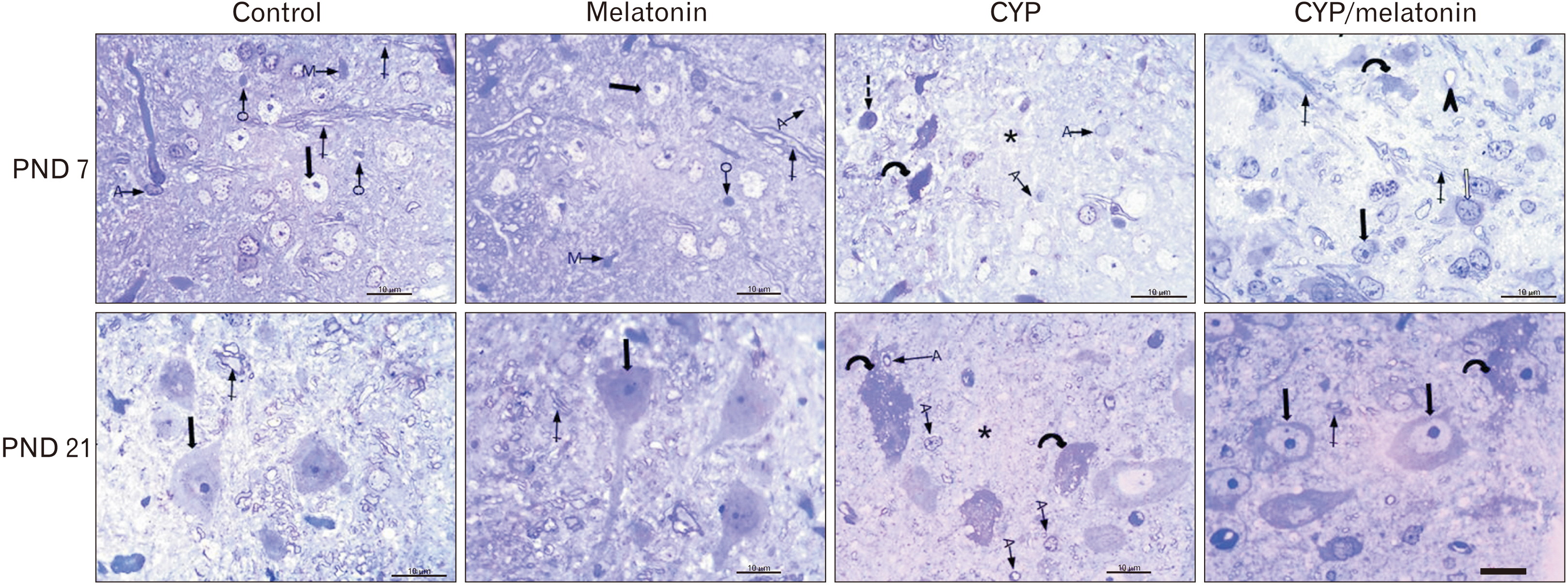
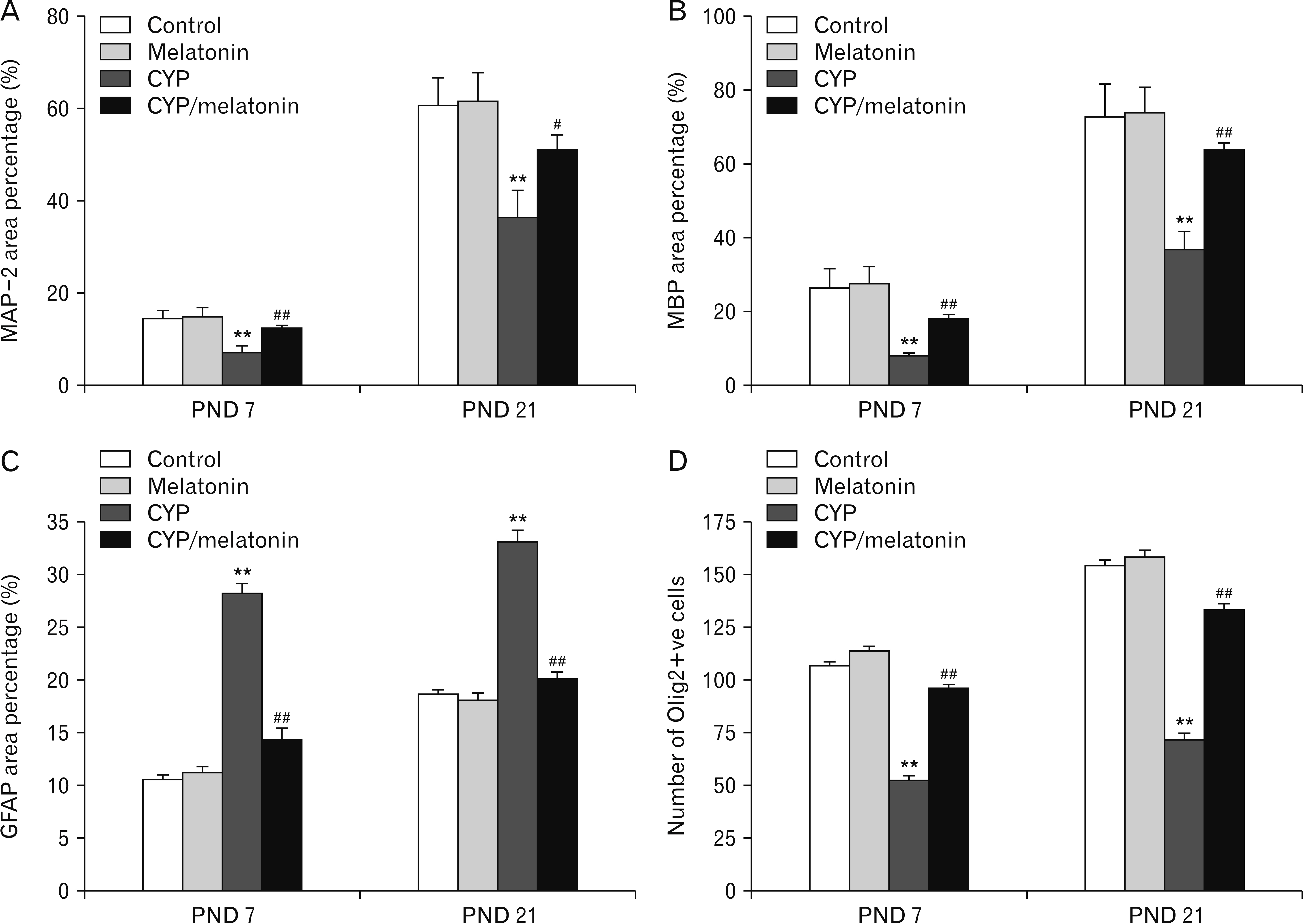
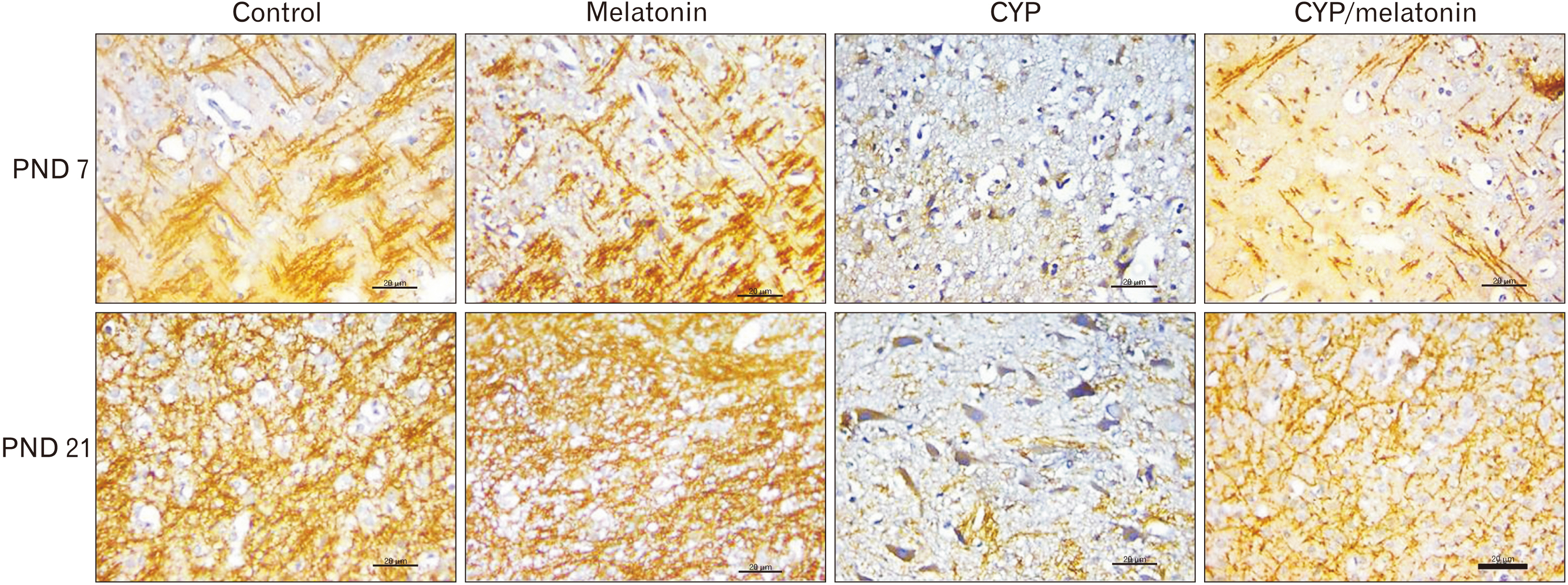
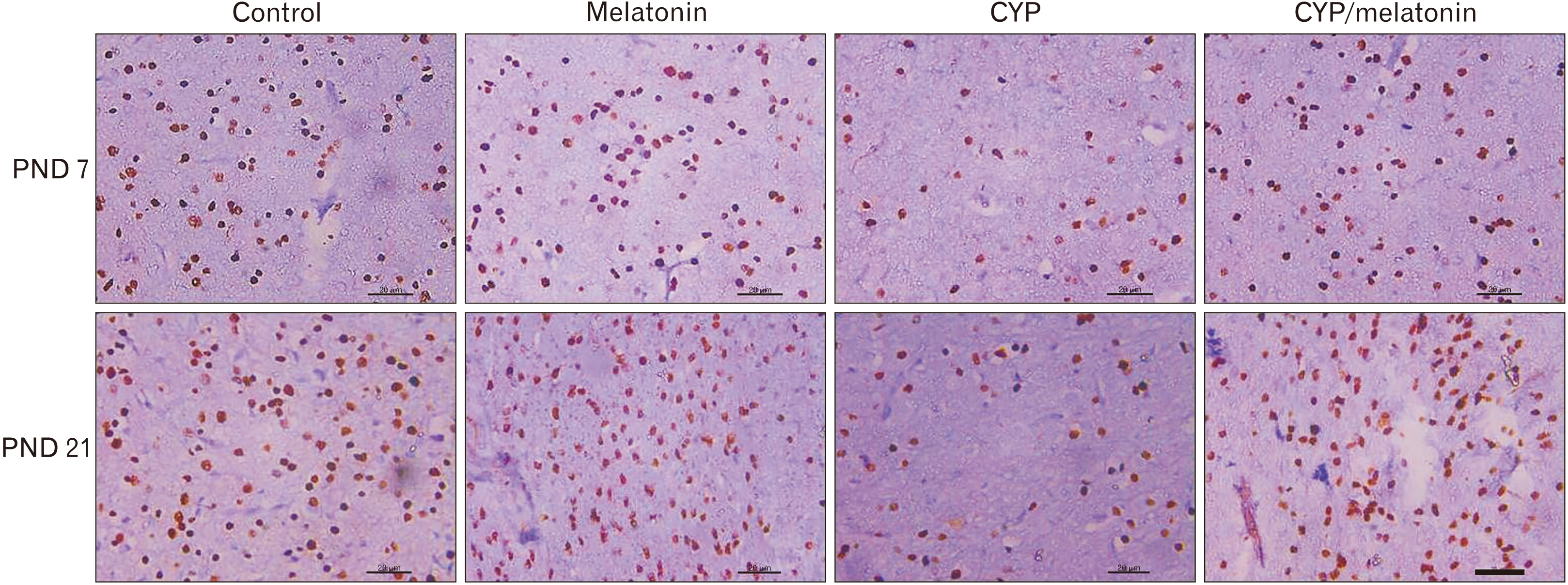
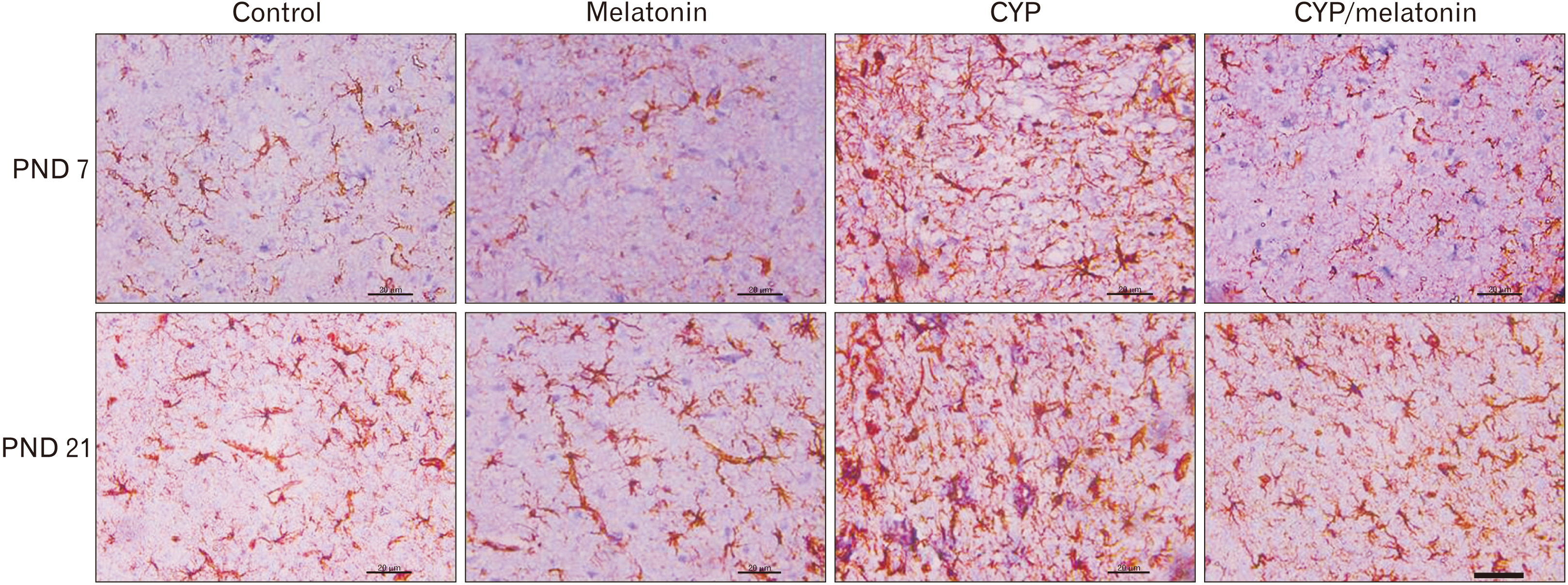
- Previous studies have shown that cypermethrin (CYP), a broad spectrum pesticide has a teratogenic effect on rat offspring born to an exposed dam with no information on its effect on the development of the brain. To the best of our knowledge, this research is the first attempt to study the postnatal development medulla oblongata of rat offspring exposed to CYP during the perinatal period and the possible neuroprotective role of melatonin. The offspring of treated female rats were organized into control, melatonin (1 mg/kg/day orally); CYP (12 mg/kg/day orally); and CYP/melatonin groups. The mothers received treatments from day 6 of gestation until day 21 after birth. At Postnatal days 7 and 21, the animals were sacrificed and their medulla oblongata was removed and subjected to histological, immunohistochemical, and electron microscopic studies. CYP induced neuronal degeneration by chromatolysis and pyknosis. Nuclear changes, cytoplasmic vacuolation, damage mitochondria, and breakdown of RER were also detected. Reduction of microtubule-associated protein-2 (MAP-2), myelin basic protein (MBP), and oligodendrocyte transcription factor expressions and increment of glial fibrillary acidic protein expression in the medulla oblongata of the developing rats were observed. On the other hand, melatonin led to an obvious improvement of the injured medulla oblongata tissues and ameliorating the damaging effects of CYP. In conclusion, melatonin has protected rats against CYP-induced histopathological and immunohistochemical changes. This may be due to the protection of MAP-2, conservation of MBP, an increment of oligodendrocytes, and alleviation of astrogliosis.
Figure
Reference
-
References
1. Sankar P, Telang AG, Manimaran A. 2011; Effect of piperine on cypermethrin-induced oxidative damage in rats. J Vet Sci Technol. 2:105. DOI: 10.4172/2157-7579.1000105.
Article2. Sankar P, Telang AG, Manimaran A. 2010; Curcumin protects against cypermethrin-induced genotoxicity in rats. Environ Toxicol Pharmacol. 30:289–91. DOI: 10.1016/j.etap.2010.07.005. PMID: 21787662.
Article3. Hussien HM, Abdou HM, Yousef MI. 2013; Cypermethrin induced damage in genomic DNA and histopathological changes in brain and haematotoxicity in rats: the protective effect of sesame oil. Brain Res Bull. 92:76–83. DOI: 10.1016/j.brainresbull.2011.10.020. PMID: 22085743.
Article4. Huang F, Liu Q, Xie S, Xu J, Huang B, Wu Y, Xia D. 2016; Cypermethrin induces macrophages death through cell cycle arrest and oxidative stress-mediated JNK/ERK signaling regulated apoptosis. Int J Mol Sci. 17:885. DOI: 10.3390/ijms17060885. PMID: 27322250. PMCID: PMC4926419.
Article5. Ferrari CKB. 2000; Free radicals, lipid peroxidation and antioxidants in apoptosis: implications in cancer, cardiovascular and neurological diseases. Biologia. 55:581–90.6. Sánchez A, Calpena AC, Clares B. 2015; Evaluating the oxidative stress in inflammation: role of melatonin. Int J Mol Sci. 16:16981–7004. DOI: 10.3390/ijms160816981. PMID: 26225957. PMCID: PMC4581180.
Article7. Wongprayoon P, Govitrapong P. 2015; Melatonin attenuates methamphetamine-induced neuroinflammation through the melatonin receptor in the SH-SY5Y cell line. Neurotoxicology. 50:122–30. DOI: 10.1016/j.neuro.2015.08.008. PMID: 26283214.
Article8. Kazemi M, Shokri S, Ganjkhani M, Ali R, Iraj JA. 2016; Modulation of axonal sprouting along rostro-caudal axis of dorsal hippocampus and no neuronal survival in parahippocampal cortices by long-term post-lesion melatonin administration in lithium-pilocarpine model of temporal lobe epilepsy. Anat Cell Biol. 49:21–33. DOI: 10.5115/acb.2016.49.1.21. PMID: 27051565. PMCID: PMC4819075.
Article9. Selevan SG, Kimmel CA, Mendola P. 2000; Identifying critical windows of exposure for children's health. Environ Health Perspect. 108 Suppl 3:451–5. DOI: 10.1289/ehp.00108s3451. PMID: 10852844. PMCID: PMC1637810.
Article10. Sayim F, Yavasoglu NÜK, Uyanikgil Y, Aktug H, Yavasoglu A, Turgut M. 2005; Neurotoxic effects of cypermethrin in wistar rats: a haematological, biochemical and histopathological study. J Health Sci. 51:300–307. DOI: 10.1248/jhs.51.300.
Article11. Issa NM, Al-Gholam MA. 2020; Apr. 17. The effect of N- acetylcysteine on the sensory retina of male albino rats exposed prenatally to cypermethrin. Folia Morphol (Warsz). [Epub]. https://doi.org/10.5603/FM.a2020.0043. DOI: 10.5603/FM.a2020.0043. PMID: 32301102.
Article12. Assayed ME, Khalaf AA, Salem HA. 2010; Protective effects of garlic extract and vitamin C against in vivo cypermethrin-induced cytogenetic damage in rat bone-marrow. Mutat Res. 702:1–7. DOI: 10.1016/j.mrgentox.2010.02.020. PMID: 20673810.13. Tain YL, Huang LT, Lin IC, Lau YT, Lin CY. 2010; Melatonin prevents hypertension and increased asymmetric dimethylarginine in young spontaneous hypertensive rats. J Pineal Res. 49:390–8. DOI: 10.1111/j.1600-079X.2010.00806.x. PMID: 20950359.
Article14. Huang C, Li X. 2014; Maternal cypermethrin exposure during the perinatal period impairs testicular development in C57BL male offspring. PLoS One. 9:e96781. DOI: 10.1371/journal.pone.0096781. PMID: 24810582. PMCID: PMC4014553.
Article15. Luo J, Chen G, Wei L, Qian H, Lai X, Wang D, Lv J, Yu X. 2014; Severe diffuse axon injury in chronic alcoholic rat medulla oblongata following a concussion blow. Alcohol Alcohol. 49:231–7. DOI: 10.1093/alcalc/agu009. PMID: 24595328.
Article16. Kim Suvarna, Layton CW, Bancroft JD. 2013. Bancroft's theory and practice of histological techniques. 7th ed. Churchill Livingstone;Oxford:17. Sharma P, Firdous S, Singh R. 2014; Neurotoxic effect of cypermethrin and protective role of resveratrol in Wistar rats. Int J Nutr Pharmacol Neurol Dis. 4:104–11. DOI: 10.4103/2231-0738.129598.
Article18. Elser BA, Kayali K, Dhakal R, O'Hare B, Wang K, Lehmler HJ, Stevens HE. 2020; Combined maternal exposure to cypermethrin and stress affect embryonic brain and placental outcomes in mice. Toxicol Sci. 175:182–96. DOI: 10.1093/toxsci/kfaa040. PMID: 32191333. PMCID: PMC7253216.
Article19. Dallegrave A, Pizzolato TM, Barreto F, Bica VC, Eljarrat E, Barceló D. 2018; Residue of insecticides in foodstuff and dietary exposure assessment of Brazilian citizens. Food Chem Toxicol. 115:329–35. DOI: 10.1016/j.fct.2018.03.028. PMID: 29574011.
Article20. Tarocco A, Caroccia N, Morciano G, Wieckowski MR, Ancora G, Garani G, Pinton P. 2019; Melatonin as a master regulator of cell death and inflammation: molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 10:317. DOI: 10.1038/s41419-019-1556-7. PMID: 30962427. PMCID: PMC6453953.
Article21. Grewal KK, Sandhu GS, Kaur R, Brar RS, Sandhu HS. 2010; Toxic impacts of cypermethrin on behavior and histology of certain tissues of albino rats. Toxicol Int. 17:94–8. DOI: 10.4103/0971-6580.72679. PMID: 21170254. PMCID: PMC2997464.
Article22. Tiso M, Gangemi R, Bargellesi Severi A, Pizzolitto S, Fabbi M, Risso A. 1995; Spontaneous apoptosis in human thymocytes. Am J Pathol. 147:434–44. PMID: 7639336. PMCID: PMC1869823.23. Abdul-Hamid M, Moustafa N, Abd Alla Asran AEM, Mowafy L. 2017; Cypermethrin-induced histopathological, ultrastructural and biochemical changes in liver of albino rats: the protective role of propolis and curcumin. Beni-Suef Univ J Basic Appl Sci. 6:160–73. DOI: 10.1016/j.bjbas.2017.03.002.
Article24. Wakabayashi T. 2002; Megamitochondria formation - physiology and pathology. J Cell Mol Med. 6:497–538. DOI: 10.1111/j.1582-4934.2002.tb00452.x. PMID: 12611638. PMCID: PMC6741312.
Article25. Guo C, Sun L, Chen X, Zhang D. 2013; Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 8:2003–14. DOI: 10.3969/j.issn.1673-5374.2013.21.009. PMID: 25206509. PMCID: PMC4145906.26. Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. 2015; Myelin damage and repair in pathologic CNS: challenges and prospects. Front Mol Neurosci. 8:35. DOI: 10.3389/fnmol.2015.00035. PMID: 26283909. PMCID: PMC4515562.
Article27. Matesic DF, Lin RC. 1994; Microtubule-associated protein 2 as an early indicator of ischemia-induced neurodegeneration in the gerbil forebrain. J Neurochem. 63:1012–20. DOI: 10.1046/j.1471-4159.1994.63031012.x. PMID: 8051544.
Article28. Guo Y, Gong HS, Zhang J, Xie WL, Tian C, Chen C, Shi Q, Wang SB, Xu Y, Zhang BY, Dong XP. 2012; Remarkable reduction of MAP2 in the brains of scrapie-infected rodents and human prion disease possibly correlated with the increase of calpain. PLoS One. 7:e30163. DOI: 10.1371/journal.pone.0030163. PMID: 22272295. PMCID: PMC3260227.
Article29. Betancourt AM, Burgess SC, Carr RL. 2006; Effect of developmental exposure to chlorpyrifos on the expression of neurotrophin growth factors and cell-specific markers in neonatal rat brain. Toxicol Sci. 92:500–6. DOI: 10.1093/toxsci/kfl004. PMID: 16675515.
Article30. Chen X, Guo C, Kong J. 2012; Oxidative stress in neurodegenerative diseases. Neural Regen Res. 7:376–85. DOI: 10.3969/j.issn.1673-5374.2012.05.009. PMID: 25774178. PMCID: PMC4350122.31. Oksanen M, Lehtonen S, Jaronen M, Goldsteins G, Hämäläinen RH, Koistinaho J. 2019; Astrocyte alterations in neurodegenerative pathologies and their modeling in human induced pluripotent stem cell platforms. Cell Mol Life Sci. 76:2739–60. DOI: 10.1007/s00018-019-03111-7. PMID: 31016348. PMCID: PMC6588647.
Article32. Mohamed HK, Eltony SA. 2020; Effect of acute pentylenetetrazol injection induced epileptic seizures on rat dentate gyrus at different postnatal ages. Anat Cell Biol. 53:84–94. DOI: 10.5115/acb.19.083. PMID: 32274253. PMCID: PMC7118254.
Article33. Rao MV, Purohit AR. 2011; Neuroprotection by melatonin on mercury induced toxicity in the rat brain. Pharmacol Pharm. 2:375–85. DOI: 10.4236/pp.2011.24049.
Article34. Motallebzadeh E, Tameh AA, Zavareh SAT, Farhood B, Aliasgharzedeh A, Mohseni M. 2020; Apr. 23. Neuroprotective effect of melatonin on radiation-induced oxidative stress and apoptosis in the brainstem of rats. J Cell Physiol. [Epub]. https://doi.org/10.1002/jcp.29722. DOI: 10.1002/jcp.29722. PMID: 32324264.
Article35. Cheung RT. 2003; The utility of melatonin in reducing cerebral damage resulting from ischemia and reperfusion. J Pineal Res. 34:153–60. DOI: 10.1034/j.1600-079X.2003.00034.x. PMID: 12614473.
Article36. Cohen-Mansfield J, Garfinkel D, Lipson S. 2000; Melatonin for treatment of sundowning in elderly persons with dementia - a preliminary study. Arch Gerontol Geriatr. 31:65–76. DOI: 10.1016/S0167-4943(00)00068-6. PMID: 10989165.
Article37. Meléndez J, Maldonado V, Ortega A. 1996; Effect of melatonin on beta-tubulin and MAP2 expression in NIE-115 cells. Neurochem Res. 21:653–8. DOI: 10.1007/BF02527721. PMID: 8829136.38. Ghareghani M, Scavo L, Jand Y, Farhadi N, Sadeghi H, Ghanbari A, Mondello S, Arnoult D, Gharaghani S, Zibara K. 2019; Melatonin therapy modulates cerebral metabolism and enhances remyelination by increasing PDK4 in a mouse model of multiple sclerosis. Front Pharmacol. 10:147. DOI: 10.3389/fphar.2019.00147. PMID: 30873027. PMCID: PMC6403148.
Article39. Baydas G, Reiter RJ, Yasar A, Tuzcu M, Akdemir I, Nedzvetskii VS. 2003; Melatonin reduces glial reactivity in the hippocampus, cortex, and cerebellum of streptozotocin-induced diabetic rats. Free Radic Biol Med. 35:797–804. DOI: 10.1016/S0891-5849(03)00408-8. PMID: 14583344.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Melatonin Injection into Rat Brain Stem with Chronic Stress on Serotonergic Immunoreactivity
- Effect of melatonin on the onset of puberty in male juvenile rats
- The effect of treatment with tryptophan and/or reserpine on the serotonergic immunoreactivity in raphe nucleus of medulla oblongata and midbrain of the rats
- Immunocytochemical and ultrastructural study of localization of the putrescine in rat medulla oblongata
- Protective Effect of Melatonin on Neuropathy in Streptozotocin-Induced Diabetic Rats