Anat Cell Biol.
2020 Dec;53(4):451-459. 10.5115/acb.20.081.
Cervical nerve roots and the dural sheath: a histological study using human fetuses near term
- Affiliations
-
- 1Department of Histology and Embryology, Tokyo Dental College, Tokyo, Japan
- 2Department of Anatomy, Tokyo Dental College, Tokyo, Japan
- 3Division of Internal Medicine, Jikou-kai Home Visit Clinic, Sapporo, Japan
- KMID: 2509691
- DOI: http://doi.org/10.5115/acb.20.081
Abstract
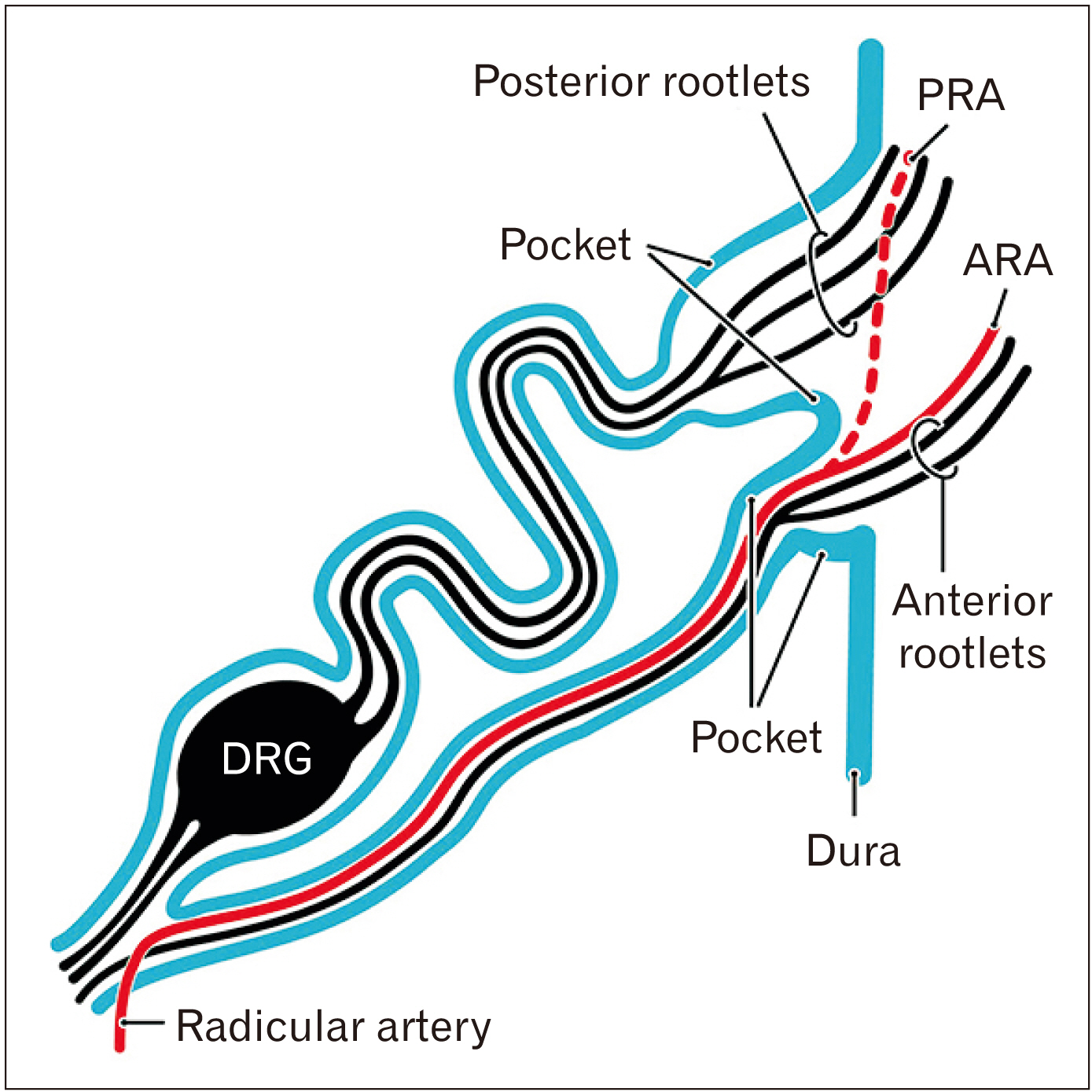
- We have previously reported that the thoracolumbar posterior nerve root shows a tortuous epidural course, based on studies of human fetuses near term. For comparison with the cervical nerve, examinations were conducted using frontal, sagittal and horizontal sections of cervical vertebrae from 22 fetuses at 30–38 weeks of gestation. The cervical nerve root showed a short, straight and lateral course near the zygapophysial joint. Multiple rather than single bundles of the cervical posterior root seemed to account for the majority of sensory nerve fibers innervating the upper extremity. Fasciculation of rootlets was evident near the thoracolumbar spinal cord, whereas it was seen in the dural pocket at the nerve exit from the dural sac although both sites were subdural. As in the thoracolumbar region, the nerve sheath was continuous with the dura mater and independently surrounded each of the anterior and posterior roots. Radicular arteries were few in the cervical region. In 2 of the 22 fetuses (31 weeks and 33 weeks), there was a segmental, unilateral abnormality of nerve rootlet fasciculation where the dorsal root ganglion was located lateral or peripheral to the intervertebral region. Long nerve roots running inferiorly are a necessary adaptation to the delayed and marked growth of the thoracolumbar vertebral column. In children, the cervical nerve roots are likely to be affected by movement or dislocation of the vertebrae. The segmental abnormality of the cervical nerve root may be linked to rare variations in the brachial plexus.
Keyword
Figure
Reference
-
References
1. Tubbs RS, Lobashevsky A, Oakes P, D'Antoni AV, Hattab E, Topp K, Loukas M, Spinner R. 2015; Meningeal relationships to the spinal nerves and rootlets: a gross, histological, and radiological study with application to intradural extramedullary spinal tumors. Childs Nerv Syst. 31:675–81. DOI: 10.1007/s00381-015-2648-z. PMID: 25686899.
Article2. Jang HS, Cho KH, Chang H, Jin ZW, Rodriguez-Vazquez JF, Murakami G. 2016; The filum terminale revisited: a histological study in human fetuses. Pediatr Neurosurg. 51:9–19. DOI: 10.1159/000439284. PMID: 26595116.
Article3. Cho KH, Jin ZW, Abe H, Shibata S, Murakami G, Rodríguez-Vázquez JF. 2016; Neural-dural transition at the thoracic and lumbar spinal nerve roots: a histological study of human late-stage fetuses. Biomed Res Int. 2016:8163519. DOI: 10.1155/2016/8163519. PMID: 27069926. PMCID: PMC4812201.
Article4. Yi M, Lee JW, Yeom JS, Joe E, Hong SH, Lee GY, Kang HS. 2014; C2 nerve root on magnetic resonance imaging of occipital neuralgia. Spine (Phila Pa 1976). 39:1077–83. DOI: 10.1097/BRS.0000000000000345. PMID: 24732835.
Article5. O'Rahilly R, Muller F, Meyer DB. 1980; The human vertebral column at the end of the embryonic period proper. 1. The column as a whole. J Anat. 131(Pt 3):565–75. PMID: 7216919. PMCID: PMC1233253.6. Castellana C, Kósa F. 1999; Morphology of the cervical vertebrae in the fetal-neonatal human skeleton. J Anat. 194(Pt 1):147–52. DOI: 10.1046/j.1469-7580.1999.19410147.x. PMID: 10227677. PMCID: PMC1467903.
Article7. Cattell HS, Filtzer DL. 1965; Pseudosubluxation and other normal variations in the cervical spine in children. A study of one hundred and sixty children. J Bone Joint Surg Am. 47:1295–309. DOI: 10.2106/00004623-196547070-00001. PMID: 5837630.8. Bogduk N. 2016; Functional anatomy of the spine. Handb Clin Neurol. 136:675–88. DOI: 10.1016/B978-0-444-53486-6.00032-6. PMID: 27430435.
Article9. San Román P, Palma JC, Oteo MD, Nevado E. 2002; Skeletal maturation determined by cervical vertebrae development. Eur J Orthod. 24:303–11. DOI: 10.1093/ejo/24.3.303. PMID: 12143094.10. Khorooshi MH, Fischer Hansen B, Keeling J, Nolting D, Kjaer I. 2001; Prenatal localization of the dorsal root ganglion in different segments of the normal human vertebral column. Spine (Phila Pa 1976). 26:1–5. DOI: 10.1097/00007632-200101010-00002. PMID: 11148637.
Article11. Müller F, O'Rahilly R. 1986; Somitic-vertebral correlation and vertebral levels in the human embryo. Am J Anat. 177:3–19. DOI: 10.1002/aja.1001770103. PMID: 3535481.
Article12. Müller F, O'Rahilly R. 2003; Segmentation in staged human embryos: the occipitocervical region revisited. J Anat. 203:297–315. DOI: 10.1046/j.1469-7580.2003.00219.x. PMID: 14529047. PMCID: PMC1571167.13. Hashimoto J, Murakami G, Tsugane MH, Chisaka O, Capecchi MR, Ogino T. 1999; Lumbosacral plexus in Hoxa9 knockout mice with special reference to their nerve variations identified according to whether they were interphenotypic or intergenotypic differences. Kaibogaku Zasshi. 74:609–30. PMID: 10659578.14. Bots J, Wijnaendts LC, Delen S, Van Dongen S, Heikinheimo K, Galis F. 2011; Analysis of cervical ribs in a series of human fetuses. J Anat. 219:403–9. DOI: 10.1111/j.1469-7580.2011.01400.x. PMID: 21689099. PMCID: PMC3171776.
Article15. Klika E, Zajícová A. 1976; The development of meninges in chicken embryos. Anat Anz. 140:379–86. PMID: 1023773.16. O'Rahilly R, Müller F. 1986; The meninges in human development. J Neuropathol Exp Neurol. 45:588–608. DOI: 10.1097/00005072-198609000-00008. PMID: 3746345.17. Hunter AG, Seaver LH, Stevenson RE. 2011; Limb-body wall defect. Is there a defensible hypothesis and can it explain all the associated anomalies? Am J Med Genet A. 155A:2045–59. DOI: 10.1002/ajmg.a.34161. PMID: 21815262.
Article18. Raphaeli T, Parimi C, Mattix K, Javid PJ. 2010; Acute colonic obstruction from Ladd bands: a unique complication from intestinal malrotation. J Pediatr Surg. 45:630–1. DOI: 10.1016/j.jpedsurg.2009.12.026. PMID: 20223332.
Article19. Komuro H, Hoshino N, Urita Y, Fujishiro J, Sakamoto N, Ono K, Kaneko M. 2010; Pathogenic implications of remnant vitelline structures in gastroschisis. J Pediatr Surg. 45:2025–9. DOI: 10.1016/j.jpedsurg.2010.04.017. PMID: 20920723.
Article20. Fukuzawa R, Toma M, Nomura A. 2011; Histology of a paraumbilical band in a neonate with gastroschisis. Pediatr Dev Pathol. 14:493–5. DOI: 10.2350/11-06-1053-CR.1. PMID: 21875339.
Article21. Kim JH, Hwang SE, Rodríguez-Vázquez JF, Murakami G, Cho BH. 2014; Liver agenesis with omphalocele: a report of two human embryos using serial histological sections. Pediatr Dev Pathol. 17:431–40. DOI: 10.2350/14-05-1484-OA.1. PMID: 25133969.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Avulsion Injury of Lumbosacral Nerve Roots Associated with Femur Fractures: A case Report
- Primary Malignant Melanoma of the Cervical Spinal Nerve Root
- An Anatomical Study on the Variations of the First Cervical Dorsal Root
- Two Cases of Neurilemmoma of the Cervical Vagus Nerve Including IntracapsularEnucleation of Nerve Preservation
- Neurilemmoma in Cervical Dorsal Nerve Root: A case report