Anat Cell Biol.
2020 Dec;53(4):405-410. 10.5115/acb.20.202.
Fetal development of the human trapezius and sternocleidomastoid muscles
- Affiliations
-
- 1Department of Neurology, Wonkwang University School of Medicine and Hospital, Institute of Wonkwang Medical Science, Iksan, Korea
- 2Department of Anatomy, Tokyo Dental College, Tokyo, Japan
- 3Division of Internal Medicine, Jikou-kai Clinic of Home Visits, Sapporo, Japan
- 4Department of Anatomy and Human Embryology, Institute of Embryology, Faculty of Medicine, Complutense University, Madrid, Spain
- KMID: 2509685
- DOI: http://doi.org/10.5115/acb.20.202
Abstract
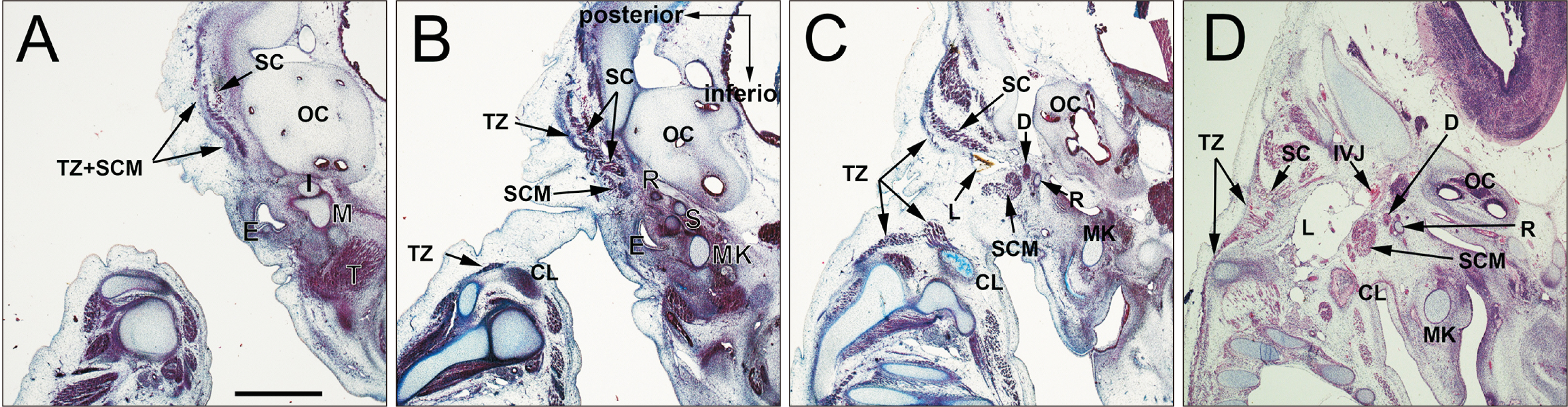
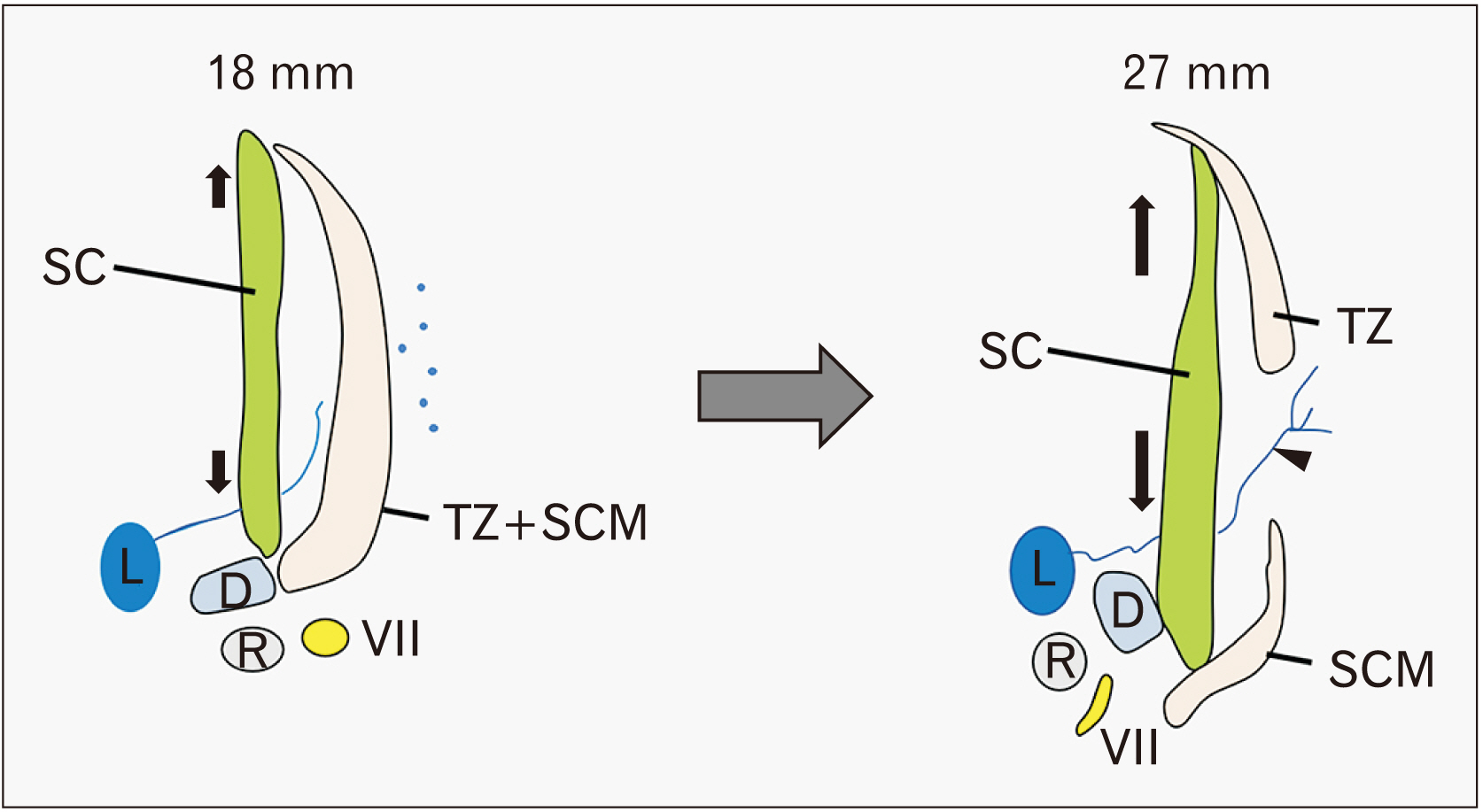
- At present, there is no photographic evidence of splitting of the trapezius and sternocleidomastoid muscles (SCMs), which share a common anlage that extends caudally toward the limb bud in the embryo at a length of 9 mm. Therefore, the aim of the present study was to identify which structures divide the caudal end of the common anlage at the first sign of splitting into two muscles. In 11 mm-long specimens, the SCM and trapezius muscles were identified as a single mesenchymal condensation. In 15 and 18 mm-long specimens, the SCM and trapezius muscles were separated and extended posteriorly and lymphatic tissues appeared in a primitive lateral cervical space surrounded by the SCM (anterior). In 21 mm-long specimens, the lymphatic vessels were dilated and the accompanying afferents were forming connections with the subcutaneous tissue through a space between the SCM and trapezius muscles. In 27 mm-long specimens, cutaneous lymphatic vessels were evident and had entered the deep tissue between the SCM and trapezius muscles. Vascular dilation may be viewed as a result of less mechanical stress or pressure after muscle splitting.
Keyword
Figure
Reference
-
References
1. Fujita T. 1959; The smaller occipital nerve, its topographic relation to the trapezius-sternocleidomastoideus muscle system. Okajimas Folia Anat Jpn. 33:217–24. DOI: 10.2535/ofaj1936.33.4_217.
Article2. Kuratani S. 2008; Evolutionary developmental studies of cyclostomes and the origin of the vertebrate neck. Dev Growth Differ. 50(Suppl 1):S189–94. DOI: 10.1111/j.1440-169X.2008.00985.x. PMID: 18430164.
Article3. Matsuoka T, Ahlberg PE, Kessaris N, Iannarelli P, Dennehy U, Richardson WD, McMahon AP, Koentges G. 2005; Neural crest origins of the neck and shoulder. Nature. 436:347–55. DOI: 10.1038/nature03837. PMID: 16034409. PMCID: PMC1352163.
Article4. Pu Q, Patel K, Huang R. 2015; The lateral plate mesoderm: a novel source of skeletal muscle. Results Probl Cell Differ. 56:143–63. DOI: 10.1007/978-3-662-44608-9_7. PMID: 25344670.
Article5. Keibel F, Mall FP. 1910. Manual of human embryology. J.B. Lippincott Company;Philadelphia:6. Mekonen HK, Hikspoors JP, Mommen G, Eleonore KÖhler S, Lamers WH. 2016; Development of the epaxial muscles in the human embryo. Clin Anat. 29:1031–45. DOI: 10.1002/ca.22775. PMID: 27571325.
Article7. Yamamoto M, Hashimoto K, Honkura Y, Murakami G, Abe H, Rodríguez-Vázquez JF, Abe SI. 2020; Morphology of the upper esophageal sphincter or cricopharyngeus muscle revisited: a study using adult and fetal specimens. Clin Anat. 33:782–94. DOI: 10.1002/ca.23506. PMID: 31659797.8. Kitamura K, Cho KH, Yamamoto M, Ishii M, Murakami G, Rodríguez-Vázquez JF, Abe SI. 2019; Suboccipital myodural bridges revisited: application to cervicogenic headaches. Clin Anat. 32:914–28. DOI: 10.1002/ca.23411. PMID: 31116454.
Article9. Sakanaka K, Yamamoto M, Hirouchi H, Kim JH, Murakami G, Rodríguez Vázquez JF, Abe SI. 2019; A temporary disc-like structure at the median atlanto-axial joint in human fetuses. Anat Cell Biol. 52:436–42. DOI: 10.5115/acb.19.128. PMID: 31949983. PMCID: PMC6952699.
Article10. Jin ZW, Cho KH, Abe H, Katori Y, Murakami G, Rodríguez-Vázquez JF. 2017; Fetal facial nerve course in the ear region revisited. Surg Radiol Anat. 39:885–95. DOI: 10.1007/s00276-017-1818-y. PMID: 28194509.
Article11. Katori Y, Hyun Kim J, Rodríguez-Vázquez JF, Kawase T, Murakami G, Hwan Cho B. 2011; Early fetal development of the intermediate tendon of the human digastricus and omohyoideus muscles: a critical difference in histogenesis. Clin Anat. 24:843–52. DOI: 10.1002/ca.21182. PMID: 21538565.
Article12. Nagakura R, Yamamoto M, Jeong J, Hinata N, Katori Y, Chang WJ, Abe S. 2020; Switching of Sox9 expression during musculoskeletal system development. Sci Rep. 10:8425. DOI: 10.1038/s41598-020-65339-9. PMID: 32439983. PMCID: PMC7242482.
Article13. Yamamoto M, Abe S. 2020; Mechanism of muscle-tendon-bone complex development in the head. Anat Sci Int. 95:165–73. DOI: 10.1007/s12565-019-00523-0. PMID: 31916224.
Article14. Yamamoto M, Takada H, Ishizuka S, Kitamura K, Jeong J, Sato M, Hinata N, Abe S. 2020; Morphological association between the muscles and bones in the craniofacial region. PLoS One. 15:e0227301. DOI: 10.1371/journal.pone.0227301. PMID: 31923241. PMCID: PMC6953862.
Article15. Cho KH, Cheong JS, Ha YS, Cho BH, Murakami G, Katori Y. 2012; The anatomy of fetal peripheral lymphatic vessels in the head-and-neck region: an immunohistochemical study. J Anat. 220:102–11. DOI: 10.1111/j.1469-7580.2011.01441.x. PMID: 22034965. PMCID: PMC3248668.
Article16. Haak MC, van Vugt JM. 2003; Echocardiography in early pregnancy: review of literature. J Ultrasound Med. 22:271–80. DOI: 10.7863/jum.2003.22.3.271. PMID: 12636327.17. de Mooij YM, van den Akker NM, Bekker MN, Bartelings MM, Wisse LJ, van Vugt JM, Gittenberger-de Groot AC. 2009; Abnormal Shh and FOXC2 expression correlates with aberrant lymphatic development in human fetuses with increased nuchal translucency. Prenat Diagn. 29:840–6. DOI: 10.1002/pd.2316. PMID: 19548265.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of Upper Trapezius and Sternocleidomastoid Muscle Activation according to Cervical Flexion Angle in Sitting Posture
- Studies on the Neck and Shoulder Pain
- Untrapped: bilateral hypoplasia of the trapezius muscle
- Spinal Accessory Neuropathy Secondary to Diffuse Large B-Cell Lymphoma
- Critical Illness Neuromyopathy Involving the Unilateral Lower Cranial Nerves: A Case Report