Korean J Physiol Pharmacol.
2021 Jan;25(1):15-26. 10.4196/kjpp.2021.25.1.15.
Whitening effect of novel peptide mixture by regulating melanosome biogenesis, transfer and degradation
- Affiliations
-
- 1Caregen R&D Center, Anyang 14119, Korea
- KMID: 2509651
- DOI: http://doi.org/10.4196/kjpp.2021.25.1.15
Abstract
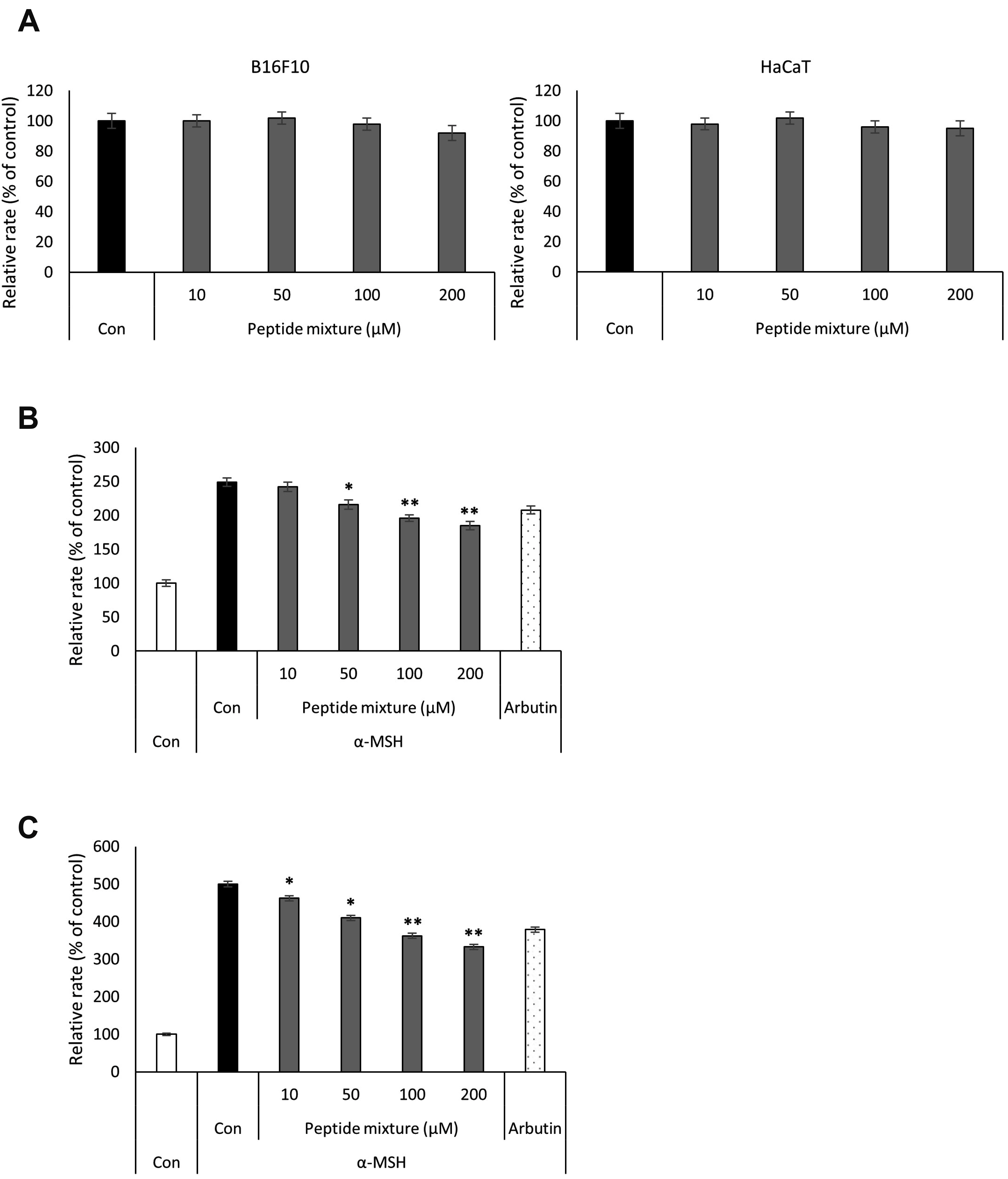
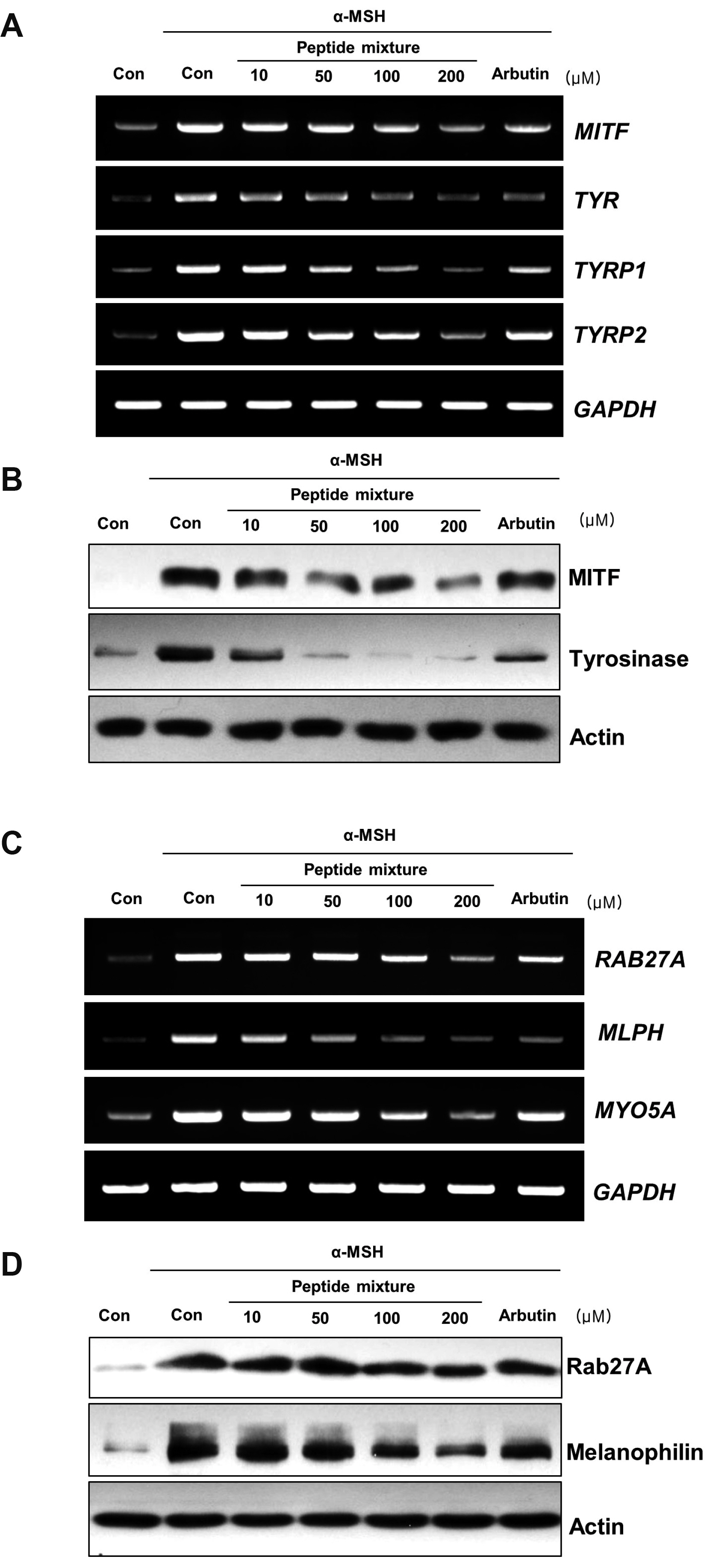
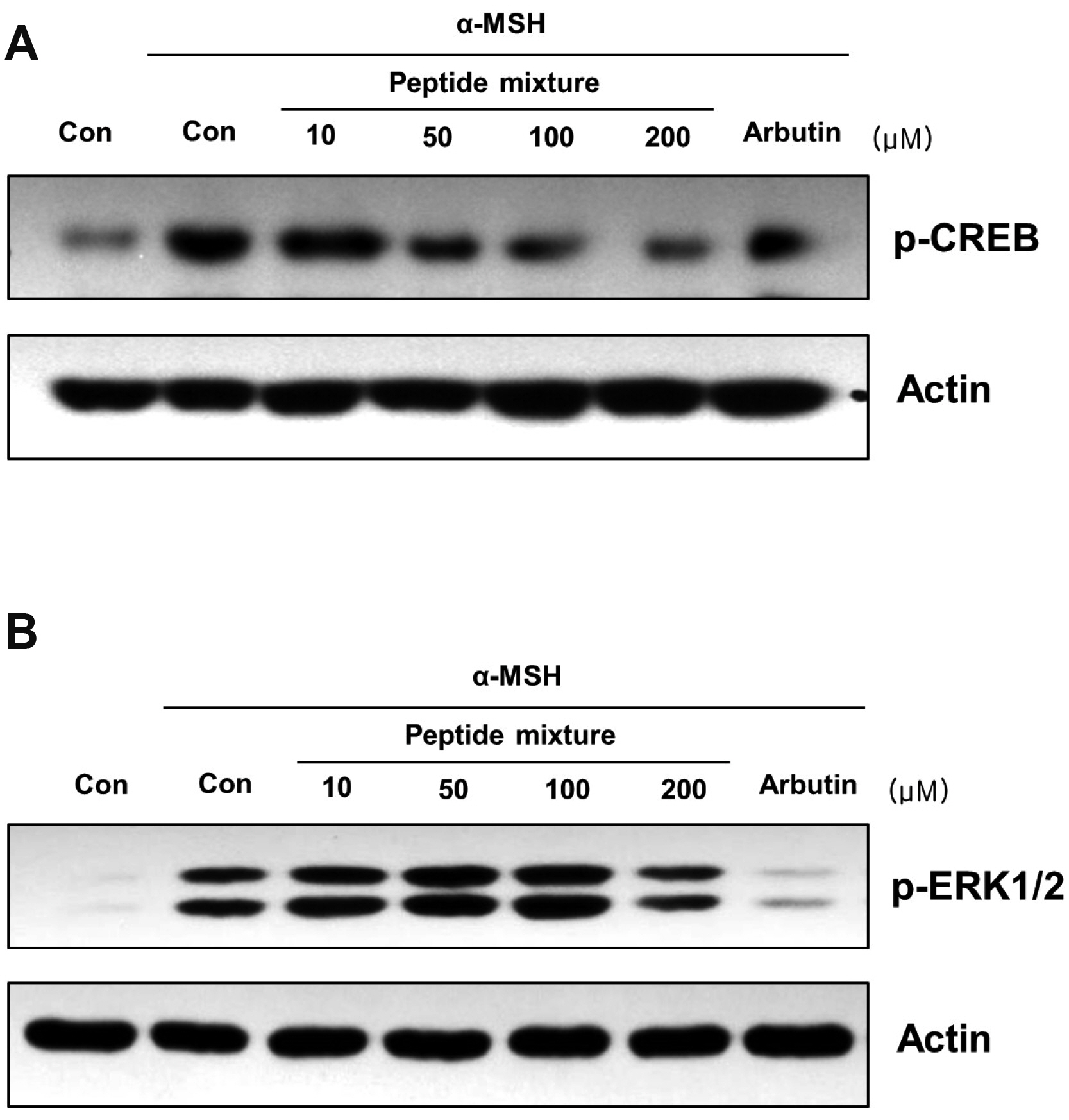
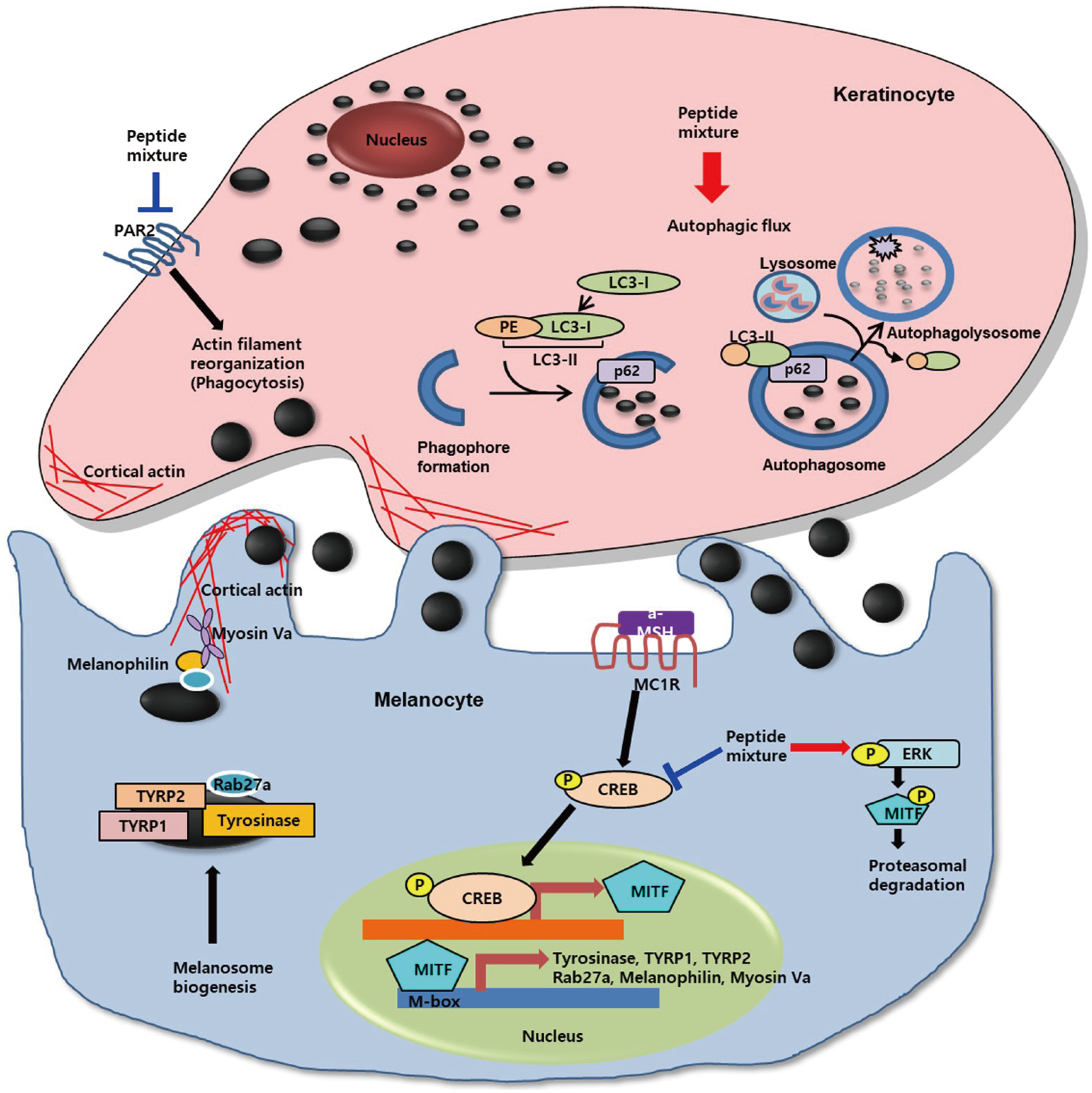
- Peptides are short chain of amino acids linked by peptide bonds. They are widely used as effective and biocompatible active ingredients in cosmetic industry. In this study, we developed novel peptide mixture and identified its anti-pigmentation effect on melanocytes and keratinocytes. Our results revealed that peptide mixture inhibited melanosome biogenesis through the regulation of microphthalmia-associated transcription factor, a key factor of melanogenesis in melanocytes. And we observed that peptide mixture inhibited melanosome uptake through the reduction of protease-activated receptor 2, a phagocytosis-related receptor in keratinocytes. Furthermore, peptide mixture activated autophagy system resulting in degradation of transferred melanosomes in keratinocytes. The anti-pigmentation effect of multi-targeting peptide mixture was assessed in a human skin equivalent model (MelanoDerm). Melanin contents in epidermal layer were significantly decreased by topical treatment of peptide mixture, suggesting that it can be applied as a novel cosmetics material having a whitening function.
Figure
Reference
-
1. Hirobe T. 2014; Keratinocytes regulate the function of melanocytes. Dermatol Sin. 32:200–204. DOI: 10.1016/j.dsi.2014.05.002.
Article2. Hachiya A, Kobayashi A, Ohuchi A, Takema Y, Imokawa G. 2001; The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J Invest Dermatol. 116:578–586. DOI: 10.1046/j.1523-1747.2001.01290.x. PMID: 11286626.
Article3. Hirobe T, Hasegawa K, Furuya R, Fujiwara R, Sato K. 2013; Effects of fibroblast-derived factors on the proliferation and differentiation of human melanocytes in culture. J Dermatol Sci. 71:45–57. DOI: 10.1016/j.jdermsci.2013.03.012. PMID: 23726358.
Article4. Schauer E, Trautinger F, Köck A, Schwarz A, Bhardwaj R, Simon M, Ansel JC, Schwarz T, Luger TA. 1994; Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 93:2258–2262. DOI: 10.1172/JCI117224. PMID: 8182158. PMCID: PMC294380.
Article5. Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek JM, Ichihashi M. 1996; Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochim Biophys Acta. 1313:130–138. DOI: 10.1016/0167-4889(96)00063-8. PMID: 8781560.
Article6. Wakamatsu K, Graham A, Cook D, Thody AJ. 1997; Characterisation of ACTH peptides in human skin and their activation of the melanocortin-1 receptor. Pigment Cell Res. 10:288–297. DOI: 10.1111/j.1600-0749.1997.tb00688.x. PMID: 9359624.
Article7. Imokawa G. 2004; Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res. 17:96–110. DOI: 10.1111/j.1600-0749.2003.00126.x. PMID: 15016298.
Article8. Chakraborty A, Slominski A, Ermak G, Hwang J, Pawelek J. 1995; Ultraviolet B and melanocyte-stimulating hormone (MSH) stimulate mRNA production for alpha MSH receptors and proopiomelanocortin-derived peptides in mouse melanoma cells and transformed keratinocytes. J Invest Dermatol. 105:655–659. DOI: 10.1111/1523-1747.ep12324134. PMID: 7594638.9. Khaled M, Larribere L, Bille K, Aberdam E, Ortonne JP, Ballotti R, Bertolotto C. 2002; Glycogen synthase kinase 3beta is activated by cAMP and plays an active role in the regulation of melanogenesis. J Biol Chem. 277:33690–33697. DOI: 10.1074/jbc.M202939200. PMID: 12093801.10. Kim YM, Cho SE, Seo YK. 2016; The activation of melanogenesis by p-CREB and MITF signaling with extremely low-frequency electromagnetic fields on B16F10 melanoma. Life Sci. 162:25–32. DOI: 10.1016/j.lfs.2016.08.015. PMID: 27543340.
Article11. Kameyama K, Sakai C, Kuge S, Nishiyama S, Tomita Y, Ito S, Wakamatsu K, Hearing VJ. 1995; The expression of tyrosinase, tyrosinase-related proteins 1 and 2 (TRP1 and TRP2), the silver protein, and a melanogenic inhibitor in human melanoma cells of differing melanogenic activities. Pigment Cell Res. 8:97–104. DOI: 10.1111/j.1600-0749.1995.tb00648.x. PMID: 7659683.
Article12. Van Gele M, Geusens B, Schmitt AM, Aguilar L, Lambert J. 2008; Knockdown of myosin Va isoforms by RNAi as a tool to block melanosome transport in primary human melanocytes. J Invest Dermatol. 128:2474–2484. DOI: 10.1038/jid.2008.100. PMID: 18401430.
Article13. Ohbayashi N, Fukuda M. 2012; Role of Rab family GTPases and their effectors in melanosomal logistics. J Biochem. 151:343–351. DOI: 10.1093/jb/mvs009. PMID: 22323658.
Article14. Park JI, Lee HY, Lee JE, Myung CH, Hwang JS. 2016; Inhibitory effect of 2-methyl-naphtho[1,2,3-de]quinolin-8-one on melanosome transport and skin pigmentation. Sci Rep. 6:29189. DOI: 10.1038/srep29189. PMID: 27381646. PMCID: PMC4933902.
Article15. Oberhofer A, Spieler P, Rosenfeld Y, Stepp WL, Cleetus A, Hume AN, Mueller-Planitz F, Ökten Z. 2017; Myosin Va's adaptor protein melanophilin enforces track selection on the microtubule and actin networks in vitro. Proc Natl Acad Sci U S A. 114:E4714–E4723. DOI: 10.1073/pnas.1619473114. PMID: 28559319. PMCID: PMC5474823.
Article16. Provance DW, James TL, Mercer JA. 2002; Melanophilin, the product of the leaden locus, is required for targeting of myosin-Va to melanosomes. Traffic. 3:124–132. DOI: 10.1034/j.1600-0854.2002.030205.x. PMID: 11929602. PMCID: PMC1351229.
Article17. Strom M, Hume AN, Tarafder AK, Barkagianni E, Seabra MC. 2002; A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J Biol Chem. 277:25423–25430. DOI: 10.1074/jbc.M202574200. PMID: 11980908.18. Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, Copeland NG, Jenkins NA, Hammer JA 3rd. 2002; Identification of an organelle receptor for myosin-Va. Nat Cell Biol. 4:271–278. DOI: 10.1038/ncb760. PMID: 11887186.
Article19. Kuroda TS, Ariga H, Fukuda M. 2003; The actin-binding domain of Slac2-a/melanophilin is required for melanosome distribution in melanocytes. Mol Cell Biol. 23:5245–5255. DOI: 10.1128/MCB.23.15.5245-5255.2003. PMID: 12861011. PMCID: PMC165717.
Article20. Cardinali G, Ceccarelli S, Kovacs D, Aspite N, Lotti LV, Torrisi MR, Picardo M. 2005; Keratinocyte growth factor promotes melanosome transfer to keratinocytes. J Invest Dermatol. 125:1190–1199. DOI: 10.1111/j.0022-202X.2005.23929.x. PMID: 16354189.
Article21. Epstein JH. 1983; Photocarcinogenesis, skin cancer, and aging. J Am Acad Dermatol. 9:487–502. DOI: 10.1016/S0190-9622(83)70160-X. PMID: 6355213.
Article22. Speeckaert R, Van Gele M, Speeckaert MM, Lambert J, van Geel N. 2014; The biology of hyperpigmentation syndromes. Pigment Cell Melanoma Res. 27:512–524. DOI: 10.1111/pcmr.12235. PMID: 24612852.
Article23. Maymone MBC, Neamah HH, Secemsky EA, Vashi NA. 2018; Correlating the Dermatology Life Quality Index and Skin Discoloration Impact Evaluation Questionnaire tools in disorders of hyperpigmentation. J Dermatol. 45:361–362. DOI: 10.1111/1346-8138.14172. PMID: 29498101.
Article24. Pillaiyar T, Manickam M, Namasivayam V. 2017; Skin whitening agents: medicinal chemistry perspective of tyrosinase inhibitors. J Enzyme Inhib Med Chem. 32:403–425. DOI: 10.1080/14756366.2016.1256882. PMID: 28097901. PMCID: PMC6010116.
Article25. Juhasz MLW, Levin MK. 2018; The role of systemic treatments for skin lightening. J Cosmet Dermatol. 17:1144–1157. DOI: 10.1111/jocd.12747. PMID: 30133125.
Article26. Zhang L, Falla TJ. 2009; Cosmeceuticals and peptides. Clin Dermatol. 27:485–494. DOI: 10.1016/j.clindermatol.2009.05.013. PMID: 19695481.
Article27. Reddy B, Jow T, Hantash BM. 2012; Bioactive oligopeptides in dermatology: part I. Exp Dermatol. 21:563–568. DOI: 10.1111/j.1600-0625.2012.01528.x. PMID: 22672743.
Article28. Chiaverini C, Beuret L, Flori E, Busca R, Abbe P, Bille K, Bahadoran P, Ortonne JP, Bertolotto C, Ballotti R. 2008; Microphthalmia-associated transcription factor regulates RAB27A gene expression and controls melanosome transport. J Biol Chem. 283:12635–12642. DOI: 10.1074/jbc.M800130200. PMID: 18281284.
Article29. Alves CP, Yokoyama S, Goedert L, Pontes CLS, Sousa JF, Fisher DE, Espreafico EM. 2017; MYO5A gene is a target of MITF in melanocytes. J Invest Dermatol. 137:985–989. DOI: 10.1016/j.jid.2016.11.026. PMID: 27939378.30. Hartman ML, Czyz M. 2015; MITF in melanoma: mechanisms behind its expression and activity. Cell Mol Life Sci. 72:1249–1260. DOI: 10.1007/s00018-014-1791-0. PMID: 25433395. PMCID: PMC4363485.
Article31. Lajis AFB, Ariff AB. 2019; Discovery of new depigmenting compounds and their efficacy to treat hyperpigmentation: evidence from in vitro study. J Cosmet Dermatol. 18:703–727. DOI: 10.1111/jocd.12900. PMID: 30866156.
Article32. Garcia-Jimenez A, Teruel-Puche JA, Berna J, Rodriguez-Lopez JN, Tudela J, Garcia-Canovas F. 2017; Action of tyrosinase on alpha and beta-arbutin: a kinetic study. PLoS One. 12:e0177330. DOI: 10.1371/journal.pone.0177330. PMID: 28493937. PMCID: PMC5426667.
Article33. Yu JS, Kim AK. 2010; Effect of combination of taurine and azelaic acid on antimelanogenesis in murine melanoma cells. J Biomed Sci. 17(Suppl 1):S45. DOI: 10.1186/1423-0127-17-S1-S45. PMID: 20804622. PMCID: PMC2994384.
Article34. Lee CS, Jang WH, Park M, Jung K, Baek HS, Joo YH, Park YH, Lim KM. 2013; A novel adamantyl benzylbenzamide derivative, AP736, suppresses melanogenesis through the inhibition of cAMP-PKA-CREB-activated microphthalmia-associated transcription factor and tyrosinase expression. Exp Dermatol. 22:762–764. DOI: 10.1111/exd.12248. PMID: 24107097.
Article35. Huang HC, Chang SJ, Wu CY, Ke HJ, Chang TM. 2014; [6]-Shogaol inhibits α-MSH-induced melanogenesis through the acceleration of ERK and PI3K/Akt-mediated MITF degradation. Biomed Res Int. 2014:842569. DOI: 10.1155/2014/842569. PMID: 25045707. PMCID: PMC4090493.36. Kim B, Lee JY, Lee HY, Nam KY, Park J, Lee SM, Kim JE, Lee JD, Hwang JS. 2013; Hesperidin suppresses melanosome transport by blocking the interaction of Rab27A-melanophilin. Biomol Ther (Seoul). 21:343–348. DOI: 10.4062/biomolther.2013.032. PMID: 24244821. PMCID: PMC3825197.
Article37. Chang H, Choi H, Joo KM, Kim D, Lee TR. 2012; Manassantin B inhibits melanosome transport in melanocytes by disrupting the melanophilin-myosin Va interaction. Pigment Cell Melanoma Res. 25:765–772. DOI: 10.1111/pcmr.12002. PMID: 22863119.
Article38. Makino-Okamura C, Niki Y, Takeuchi S, Nishigori C, Declercq L, Yaroch DB, Saito N. 2014; Heparin inhibits melanosome uptake and inflammatory response coupled with phagocytosis through blocking PI3k/Akt and MEK/ERK signaling pathways in human epidermal keratinocytes. Pigment Cell Melanoma Res. 27:1063–1074. DOI: 10.1111/pcmr.12287. PMID: 24961476.
Article39. Seiberg M, Paine C, Sharlow E, Andrade-Gordon P, Costanzo M, Eisinger M, Shapiro SS. 2000; The protease-activated receptor 2 regulates pigmentation via keratinocyte-melanocyte interactions. Exp Cell Res. 254:25–32. DOI: 10.1006/excr.1999.4692. PMID: 10623462.
Article40. Murase D, Hachiya A, Takano K, Hicks R, Visscher MO, Kitahara T, Hase T, Takema Y, Yoshimori T. 2013; Autophagy has a significant role in determining skin color by regulating melanosome degradation in keratinocytes. J Invest Dermatol. 133:2416–2424. DOI: 10.1038/jid.2013.165. PMID: 23558403.
Article41. Kim ES, Shin JH, Seok SH, Kim JB, Chang H, Park SJ, Jo YK, Choi ES, Park JS, Yeom MH, Lim CS, Cho DH. 2013; Autophagy mediates anti-melanogenic activity of 3'-ODI in B16F1 melanoma cells. Biochem Biophys Res Commun. 442:165–170. DOI: 10.1016/j.bbrc.2013.11.048. PMID: 24269817.
Article42. Li L, Chen X, Gu H. 2016; The signaling involved in autophagy machinery in keratinocytes and therapeutic approaches for skin diseases. Oncotarget. 7:50682–50697. DOI: 10.18632/oncotarget.9330. PMID: 27191982. PMCID: PMC5226613.
Article43. Schagen SK. 2017; Topical peptide treatments with effective anti-aging results. Cosmetics. 4:16. DOI: 10.3390/cosmetics4020016.
Article44. Reddy BY, Jow T, Hantash BM. 2012; Bioactive oligopeptides in dermatology: part II. Exp Dermatol. 21:569–575. DOI: 10.1111/j.1600-0625.2012.01527.x. PMID: 22672721.
Article45. Strömstedt AA, Pasupuleti M, Schmidtchen A, Malmsten M. 2009; Evaluation of strategies for improving proteolytic resistance of antimicrobial peptides by using variants of EFK17, an internal segment of LL-37. Antimicrob Agents Chemother. 53:593–602. DOI: 10.1128/AAC.00477-08. PMID: 19029324. PMCID: PMC2630634.46. Pai VV, Bhandari P, Shukla P. 2017; Topical peptides as cosmeceuticals. Indian J Dermatol Venereol Leprol. 83:9–18. DOI: 10.4103/0378-6323.186500. PMID: 27451932.
Article47. Marepally S, Boakye CH, Shah PP, Etukala JR, Vemuri A, Singh M. 2013; Design, synthesis of novel lipids as chemical permeation enhancers and development of nanoparticle system for transdermal drug delivery. PLoS One. 8:e82581. DOI: 10.1371/journal.pone.0082581. PMID: 24349315. PMCID: PMC3861410.
Article48. Kalluri H, Banga AK. 2011; Transdermal delivery of proteins. AAPS PharmSciTech. 12:431–441. DOI: 10.1208/s12249-011-9601-6. PMID: 21369712. PMCID: PMC3066337.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Simple Assay Method for Melanosome Transfer
- Inhibition of Melanosome Transport by Inducing Exon Skipping in Melanophilin
- Melanosome in Retinal Pigment Epithelium: Distribution, Type and Activity of Acid Phosphatase
- Small Non-coding Transfer RNA-Derived RNA Fragments (tRFs): Their Biogenesis, Function and Implication in Human Diseases
- Evaluation of the whitening and remineralization effects of a mixture of amorphous calcium phosphate, hydroxyapatite and tetrasodium pyrophosphate on bovine enamel