J Korean Med Sci.
2020 Dec;35(49):e432. 10.3346/jkms.2020.35.e432.
Respiratory Specimen Collection Booth for COVID-19 Test: Efficiency Based Newly Introduced Facility
- Affiliations
-
- 1Department of Infection Control, Severance Hospital, Yonsei University Health System, Seoul, Korea
- 2Department of Internal Medicine and AIDS Research Institute, Yonsei University College of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine and Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2509599
- DOI: http://doi.org/10.3346/jkms.2020.35.e432
Abstract
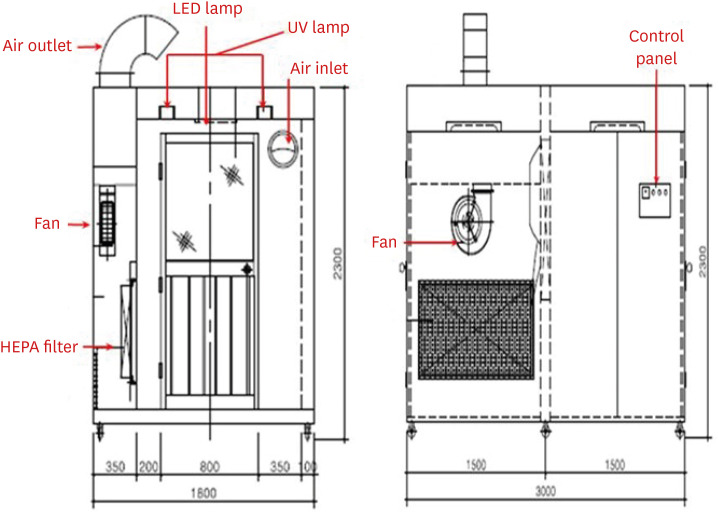
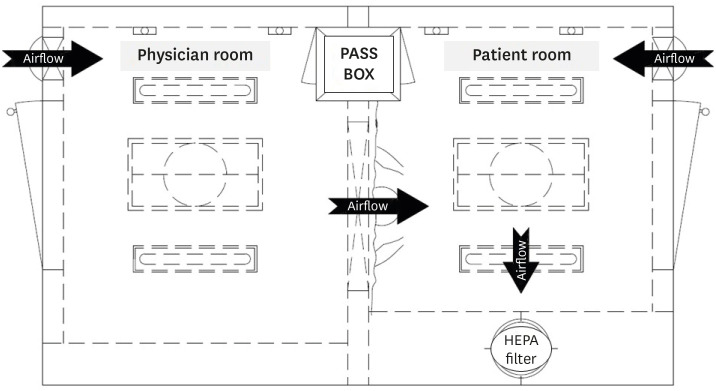
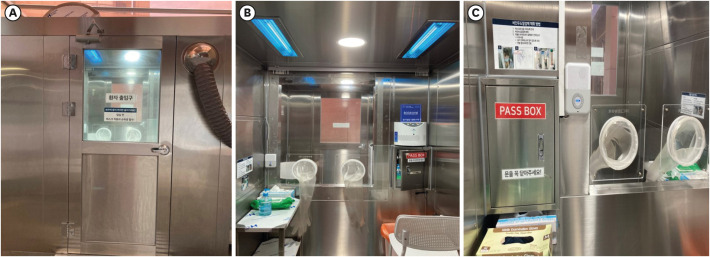
- Hospitals need to find a safe and rapid method for respiratory specimen collection as the number of patients suspicious for coronavirus disease -2019 (COVID-19) rapidly grows. Applied with significant infection control and prevention measures, a respiratory specimen collection booth was newly designed. The new respiratory specimen collection booth not only increased COVID-19 testing cases but also decreased personal protective equipment consumption.
Keyword
Figure
Reference
-
1. World Health Organization. Coronavirus Disease 2019(COVID-19) Situation Report-51. Geneva: World Health Organization;2020.2. Gao Z, Xu Y, Sun C, Wang X, Guo Y, Qiu S, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect.
Article3. Centers for Disease Control and Prevention. Guidelines for environmental infection control in health-care facilities (2003). Updated 2019. Accessed September 7, 2020. https://www.cdc.gov/infectioncontrol/guidelines/environmental/appendix/air.html.4. Cadnum JL, Li DF, Jones LD, Redmond SN, Pearlmutter B, Wilson BM, et al. Evaluation of ultraviolet-c light for rapid decontamination of airport security bins in the era of SARS-CoV-2. Pathog Immun. 2020; 5(1):133–142. PMID: 32582873.
Article5. Hong KH, Lee SW, Kim TS, Huh HJ, Lee J, Kim SY, et al. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med. 2020; 40(5):351–360. PMID: 32237288.
Article6. Kwon KT, Ko JH, Shin H, Sung M, Kim JY. Drive-through screening center for COVID-19: a safe and efficient screening system against massive community outbreak. J Korean Med Sci. 2020; 35(11):e123. PMID: 32193904.
Article7. Goodell JW. COVID-19 and finance: agendas for future research. Finance Res Lett. 2020; 35:101512.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Are We Truly Safe? Unfolding the Final Chapters of COVID-19 Walk-Through Booths
- Inconsistent Polymerase Chain Reaction Test Results From the Upper And Lower Airways of a Patient Who Underwent Total Laryngectomy During the Incubation Period for Coronavirus Disease
- Atypical modes of COVID-19 transmission: how likely are they?
- Stroke in a Young Age COVID-19 Patient: Vasculitis Feature and Changes in Cerebral Vessel Stenosis
- Causes and Clinical Relevance of Inconclusive SARS-CoV-2 Real-Time Reverse TranscriptionPCR Test Results