J Cardiovasc Imaging.
2020 Apr;28(2):79-93. 10.4250/jcvi.2019.0103.
Myocardial Positron Emission Tomography for Evaluation of Cardiac Sarcoidosis: Specialized Protocols for Better Diagnosis
- Affiliations
-
- 1Department of Cardiology, Cardiovascular Center, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Nuclear Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea
- 4Department of Nuclear Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 5Institute of Radiation Medicine, Medical Research Center, Seoul National University, Seoul, Korea
- KMID: 2509451
- DOI: http://doi.org/10.4250/jcvi.2019.0103
Abstract
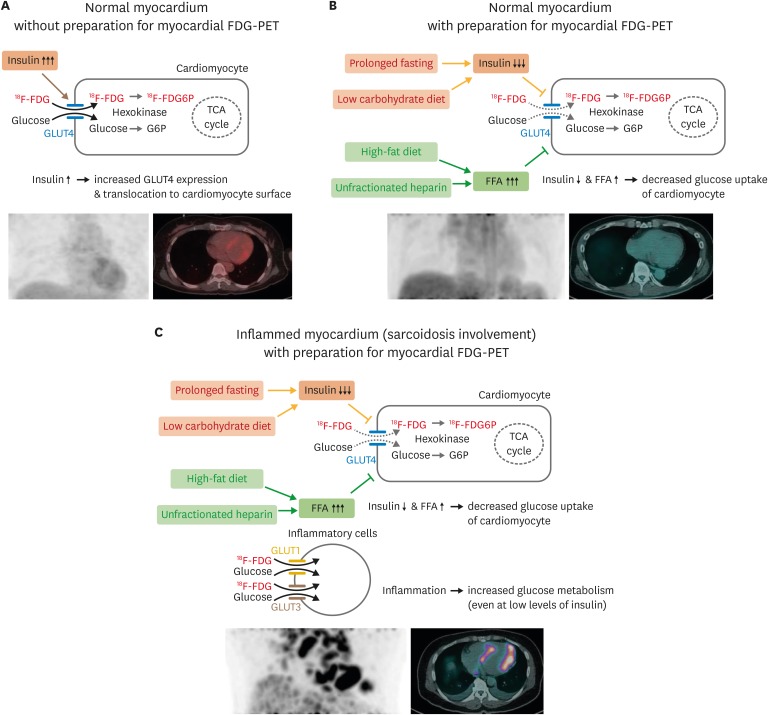
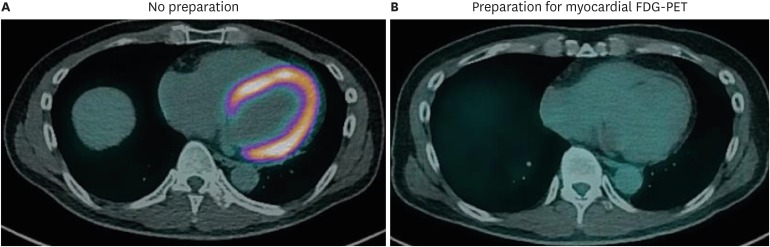
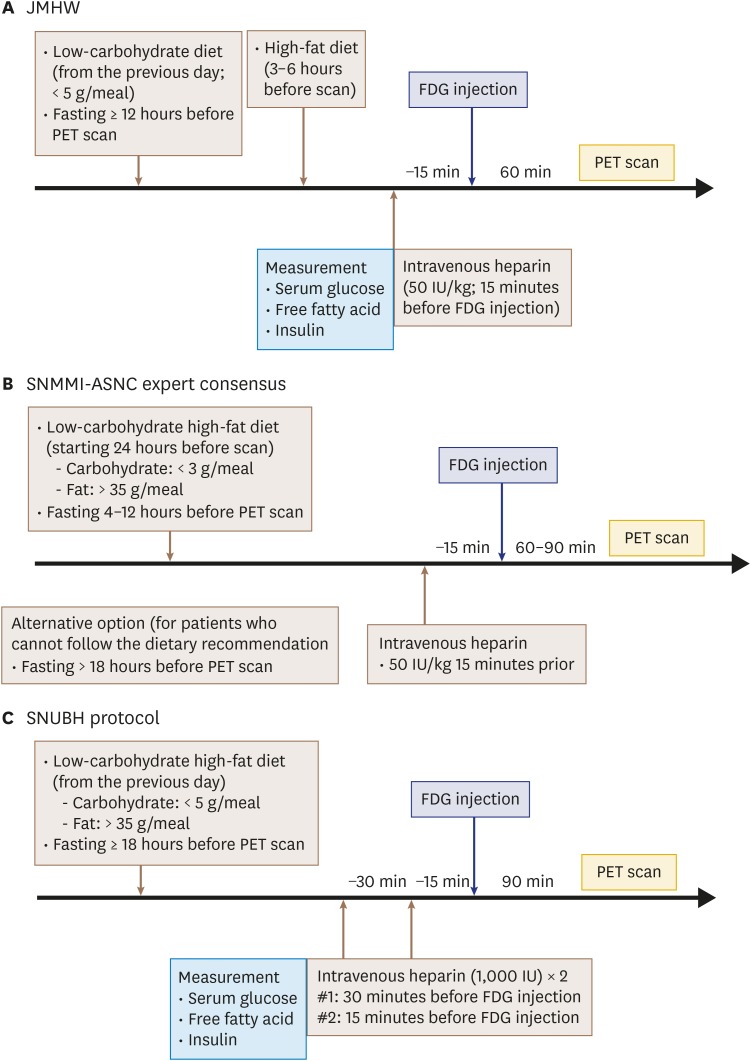
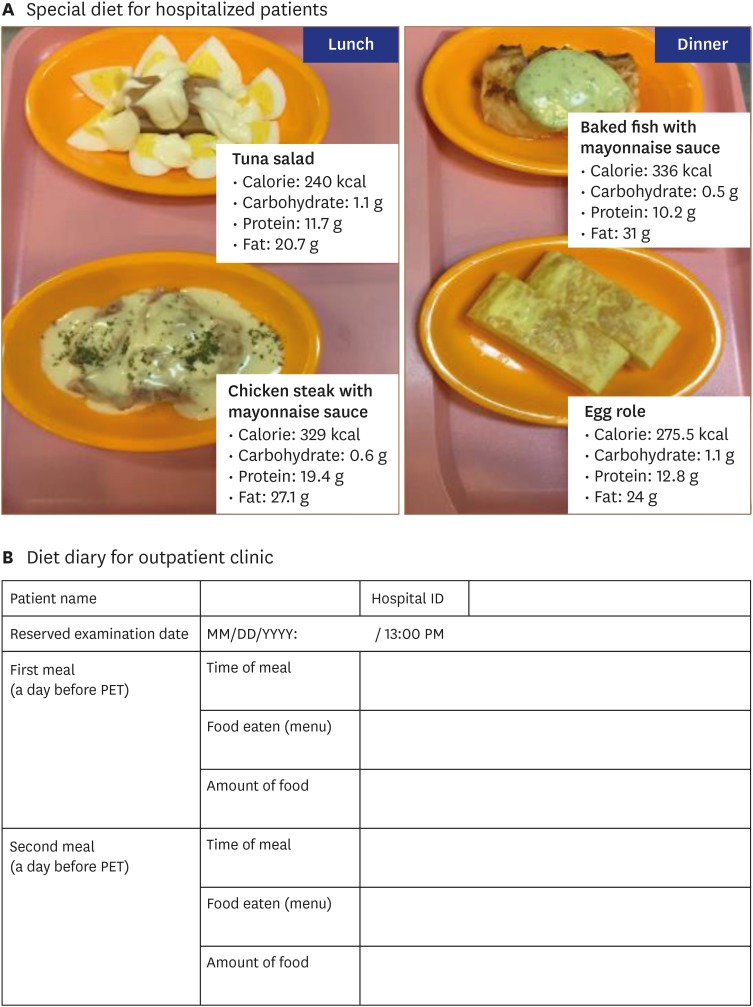
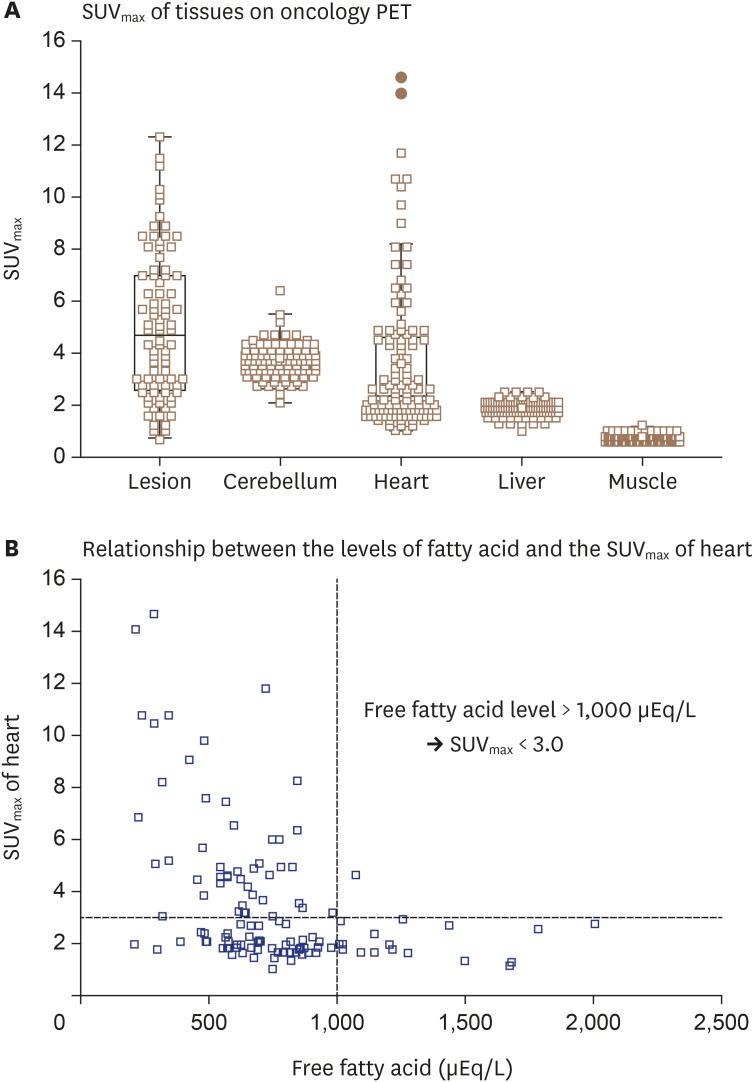
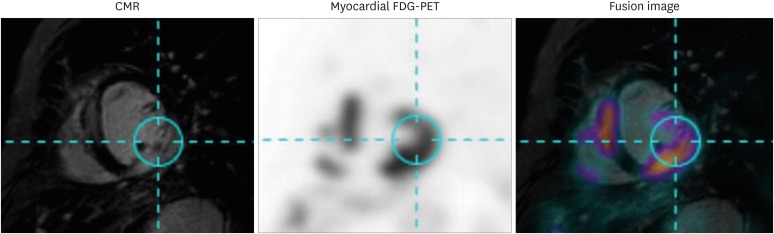
- Sarcoidosis is a multisystemic granulomatous disease of unknown etiology with various clinical presentations depending on the organs involved. Since cardiac sarcoidosis (CS) portends a higher risk of morbidity and mortality, early diagnosis and aggressive medical treatment are essential to improve the prognosis. 18F-Fluorodeoxyglucose (FDG) positron emission tomography (PET) has emerged as an important tool with practical advantages in assessing disease activity and monitoring the treatment response in patients with CS. While it has high sensitivity, it also has great variability in specificity, probably due to normal physiologic myocardial FDG uptake, which interferes with the evaluation and follow-up of CS using FDG-PET. This review details the technical aspects of FDG-PET imaging for evaluating and diagnosing CS, assessing disease activity, and monitoring therapeutic response.
Keyword
Figure
Reference
-
1. Porter GH. Sarcoid heart disease. N Engl J Med. 1960; 263:1350–1357. PMID: 13737273.2. Birnie DH, Nery PB, Ha AC, Beanlands RS. Cardiac Sarcoidosis. J Am Coll Cardiol. 2016; 68:411–421. PMID: 27443438.3. Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978; 58:1204–1211. PMID: 709777.4. Bennett MK, Gilotra NA, Harrington C, et al. Evaluation of the role of endomyocardial biopsy in 851 patients with unexplained heart failure from 2000-2009. Circ Heart Fail. 2013; 6:676–684. PMID: 23733916.5. Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014; 11:1305–1323. PMID: 24819193.6. JCS Joint Working Group. Guidelines for diagnosis and treatment of myocarditis (JCS 2009): digest version. Circ J. 2011; 75:734–743. PMID: 21304213.7. Aggarwal NR, Snipelisky D, Young PM, Gersh BJ, Cooper LT, Chareonthaitawee P. Advances in imaging for diagnosis and management of cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging. 2015; 16:949–958. PMID: 26104960.8. Blankstein R, Waller AH. Evaluation of known or suspected cardiac sarcoidosis. Circ Cardiovasc Imaging. 2016; 9:e000867. PMID: 26926267.9. Ohira H, Yoshinaga K, Manabe O, et al. Clinical application of 18F-fluorodeoxyglucose PET and LGE CMR in cardiac sarcoidosis. Ann Nucl Cardiol. 2017; 3:125–130.10. Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med. 2004; 45:1989–1998. PMID: 15585472.11. Nishiyama Y, Yamamoto Y, Fukunaga K, et al. Comparative evaluation of 18F-FDG PET and 67Ga scintigraphy in patients with sarcoidosis. J Nucl Med. 2006; 47:1571–1576. PMID: 17015889.12. Ishida Y, Yoshinaga K, Miyagawa M, et al. Recommendations for (18)F-fluorodeoxyglucose positron emission tomography imaging for cardiac sarcoidosis: Japanese Society of Nuclear Cardiology recommendations. Ann Nucl Med. 2014; 28:393–403. PMID: 24464391.13. Wisneski JA, Gertz EW, Neese RA, Mayr M. Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J Clin Invest. 1987; 79:359–366. PMID: 3805273.14. Santalucía T, Camps M, Castelló A, et al. Developmental regulation of GLUT-1 (erythroid/Hep G2) and GLUT-4 (muscle/fat) glucose transporter expression in rat heart, skeletal muscle, and brown adipose tissue. Endocrinology. 1992; 130:837–846. PMID: 1370797.15. Montessuit C, Lerch R. Regulation and dysregulation of glucose transport in cardiomyocytes. Biochim Biophys Acta. 2013; 1833:848–856. PMID: 22967513.16. Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963; 1:785–789. PMID: 13990765.17. Nuutila P, Koivisto VA, Knuuti J, et al. Glucose-free fatty acid cycle operates in human heart and skeletal muscle in vivo. J Clin Invest. 1992; 89:1767–1774. PMID: 1601987.18. Kubota K, Kubota R, Yamada S, Tada M, Takahashi T, Iwata R. Re-evaluation of myocardial FDG uptake in hyperglycemia. J Nucl Med. 1996; 37:1713–1717. PMID: 8862317.19. Yamada K, Endo S, Fukuda H, et al. Experimental studies on myocardial glucose metabolism of rats with 18F-2-fluoro-2-deoxy-D-glucose. Eur J Nucl Med. 1985; 10:341–345. PMID: 3891350.20. Miyagawa M, Yokoyama R, Nishiyama Y, Ogimoto A, Higaki J, Mochizuki T. Positron emission tomography-computed tomography for imaging of inflammatory cardiovascular diseases. Circ J. 2014; 78:1302–1310. PMID: 24817762.21. Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007; 18:1437–1446. PMID: 17301289.22. Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol. 2010; 184:4062–4068. PMID: 20368286.23. Maratou E, Dimitriadis G, Kollias A, et al. Glucose transporter expression on the plasma membrane of resting and activated white blood cells. Eur J Clin Invest. 2007; 37:282–290. PMID: 17373964.24. Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab. 2008; 295:E242–E253. PMID: 18577699.25. Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012; 53:241–248. PMID: 22228794.26. Yokoyama R, Miyagawa M, Okayama H, et al. Quantitative analysis of myocardial 18F-fluorodeoxyglucose uptake by PET/CT for detection of cardiac sarcoidosis. Int J Cardiol. 2015; 195:180–187. PMID: 26043154.27. Tahara N, Tahara A, Nitta Y, et al. Heterogeneous myocardial FDG uptake and the disease activity in cardiac sarcoidosis. JACC Cardiovasc Imaging. 2010; 3:1219–1228. PMID: 21163450.28. Sperry BW, Tamarappoo BK, Oldan JD, et al. Prognostic impact of extent, severity, and heterogeneity of abnormalities on (18)F-FDG PET scans for suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2018; 11:336–345. PMID: 28823747.29. Ahmadian A, Brogan A, Berman J, et al. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. J Nucl Cardiol. 2014; 21:925–939. PMID: 24879453.30. Blankstein R, Agarwal V. Maximizing the prognostic value of cardiac PET in patients with suspected cardiac sarcoidosis: a simple semiquantitative score may help. JACC Cardiovasc Imaging. 2018; 11:346–348. PMID: 28823739.31. Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015; 42:328–354. PMID: 25452219.32. Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer--a PET study. J Nucl Med. 1993; 34:1–6. PMID: 8418248.33. Chareonthaitawee P, Beanlands RS, Chen W, et al. Joint SNMMI-ASNC expert consensus document on the role of (18)F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Med. 2017; 58:1341–1353. PMID: 28765228.34. Morooka M, Moroi M, Uno K, et al. Long fasting is effective in inhibiting physiological myocardial 18F-FDG uptake and for evaluating active lesions of cardiac sarcoidosis. EJNMMI Res. 2014; 4:1. PMID: 24382020.35. Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. 2005; 26:1538–1543. PMID: 15809286.36. Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008; 35:933–941. PMID: 18084757.37. Langah R, Spicer K, Gebregziabher M, Gordon L. Effectiveness of prolonged fasting 18f-FDG PET-CT in the detection of cardiac sarcoidosis. J Nucl Cardiol. 2009; 16:801–810. PMID: 19548047.38. Persson E, Nordenström J, Hagenfeldt L, Nilsson-Ehle P. Plasma lipolytic activity after subcutaneous administration of heparin and a low molecular weight heparin fragment. Thromb Res. 1987; 46:697–704. PMID: 3629543.39. Persson E. Lipoprotein lipase, hepatic lipase and plasma lipolytic activity. Effects of heparin and a low molecular weight heparin fragment (Fragmin). Acta Med Scand Suppl. 1988; 724:1–56. PMID: 2843005.40. Lum DP, Wandell S, Ko J, Coel MN. Reduction of myocardial 2-deoxy-2-[18F]fluoro-D-glucose uptake artifacts in positron emission tomography using dietary carbohydrate restriction. Mol Imaging Biol. 2002; 4:232–237. PMID: 14537127.41. Cheng VY, Slomka PJ, Ahlen M, Thomson LE, Waxman AD, Berman DS. Impact of carbohydrate restriction with and without fatty acid loading on myocardial 18F-FDG uptake during PET: A randomized controlled trial. J Nucl Cardiol. 2010; 17:286–291. PMID: 20013165.42. Ohira H, Tsujino I, Yoshinaga K. ¹⁸F-Fluoro-2-deoxyglucose positron emission tomography in cardiac sarcoidosis. Eur J Nucl Med Mol Imaging. 2011; 38:1773–1783. PMID: 21559980.43. Wykrzykowska J, Lehman S, Williams G, et al. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med. 2009; 50:563–568. PMID: 19289431.44. Williams G, Kolodny GM. Suppression of myocardial 18F-FDG uptake by preparing patients with a high-fat, low-carbohydrate diet. AJR Am J Roentgenol. 2008; 190:W151-6. PMID: 18212199.45. Harisankar CN, Mittal BR, Agrawal KL, Abrar ML, Bhattacharya A. Utility of high fat and low carbohydrate diet in suppressing myocardial FDG uptake. J Nucl Cardiol. 2011; 18:926–936. PMID: 21732228.46. Demeure F, Hanin FX, Bol A, et al. A randomized trial on the optimization of 18F-FDG myocardial uptake suppression: implications for vulnerable coronary plaque imaging. J Nucl Med. 2014; 55:1629–1635. PMID: 25082852.47. Dilsizian V, Bacharach SL, Beanlands RS, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016; 23:1187–1226. PMID: 27392702.48. Hamzeh N, Steckman DA, Sauer WH, Judson MA. Pathophysiology and clinical management of cardiac sarcoidosis. Nat Rev Cardiol. 2015; 12:278–288. PMID: 25707386.49. Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014; 63:329–336. PMID: 24140661.50. Yamagishi H, Shirai N, Takagi M, et al. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J Nucl Med. 2003; 44:1030–1036. PMID: 12843216.51. Tadamura E, Yamamuro M, Kubo S, et al. Images in cardiovascular medicine. Multimodality imaging of cardiac sarcoidosis before and after steroid therapy. Circulation. 2006; 113:e771–3. PMID: 16717157.52. Ahmadian A, Pawar S, Govender P, Berman J, Ruberg FL, Miller EJ. The response of FDG uptake to immunosuppressive treatment on FDG PET/CT imaging for cardiac sarcoidosis. J Nucl Cardiol. 2017; 24:413–424. PMID: 27457527.53. Lee PI, Cheng G, Alavi A. The role of serial FDG PET for assessing therapeutic response in patients with cardiac sarcoidosis. J Nucl Cardiol. 2017; 24:19–28. PMID: 27813028.54. Osborne MT, Hulten EA, Singh A, et al. Reduction in ¹⁸F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2014; 21:166–174. PMID: 24307261.55. Shelke AB, Aurangabadkar HU, Bradfield JS, Ali Z, Kumar KS, Narasimhan C. Serial FDG-PET scans help to identify steroid resistance in cardiac sarcoidosis. Int J Cardiol. 2017; 228:717–722. PMID: 27886616.56. Dweck MR, Abgral R, Trivieri MG, et al. Hybrid magnetic resonance imaging and positron emission tomography with fluorodeoxyglucose to diagnose active cardiac sarcoidosis. JACC Cardiovasc Imaging. 2018; 11:94–107. PMID: 28624396.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Myocardial Blood Flow and Coronary Flow Reserve Using Positron Emission Tomography
- Can FDG PET Serve as a Clinically Relevant Tool for Detecting Active Non‑sarcoidotic Myocarditis?
- Recent Advances in Nuclear Cardiology
- Emerging Tracers for Nuclear Cardiac PET Imaging
- Cardiac Sarcoidosis Presenting With Complete Atrioventricular Block and Sustained Monomorphic Ventricular Tachycardia