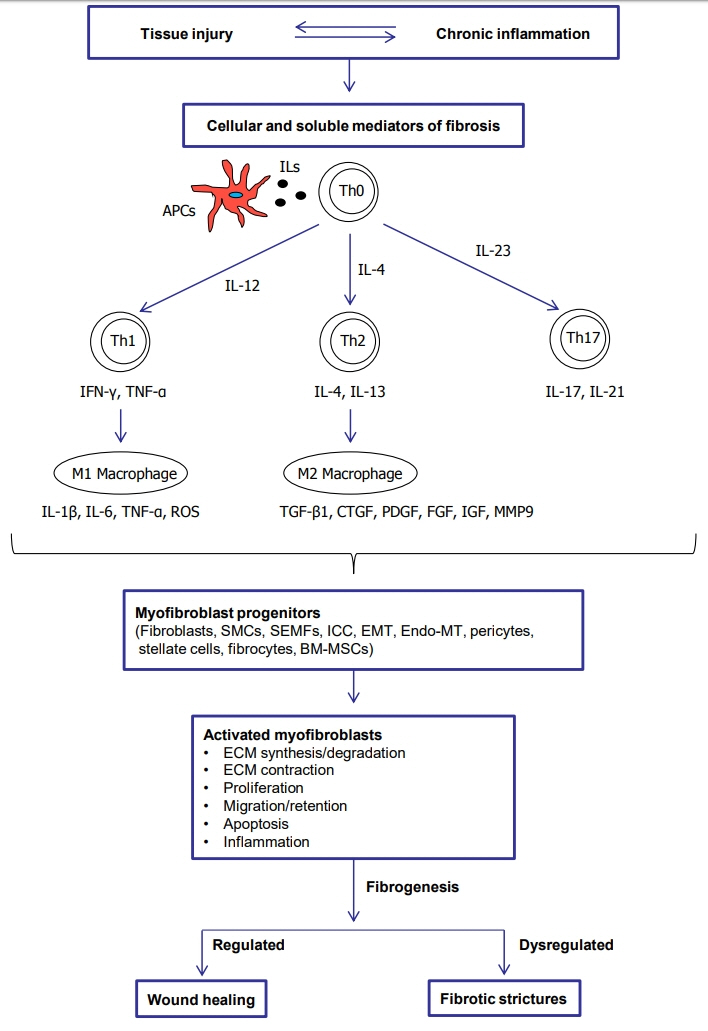
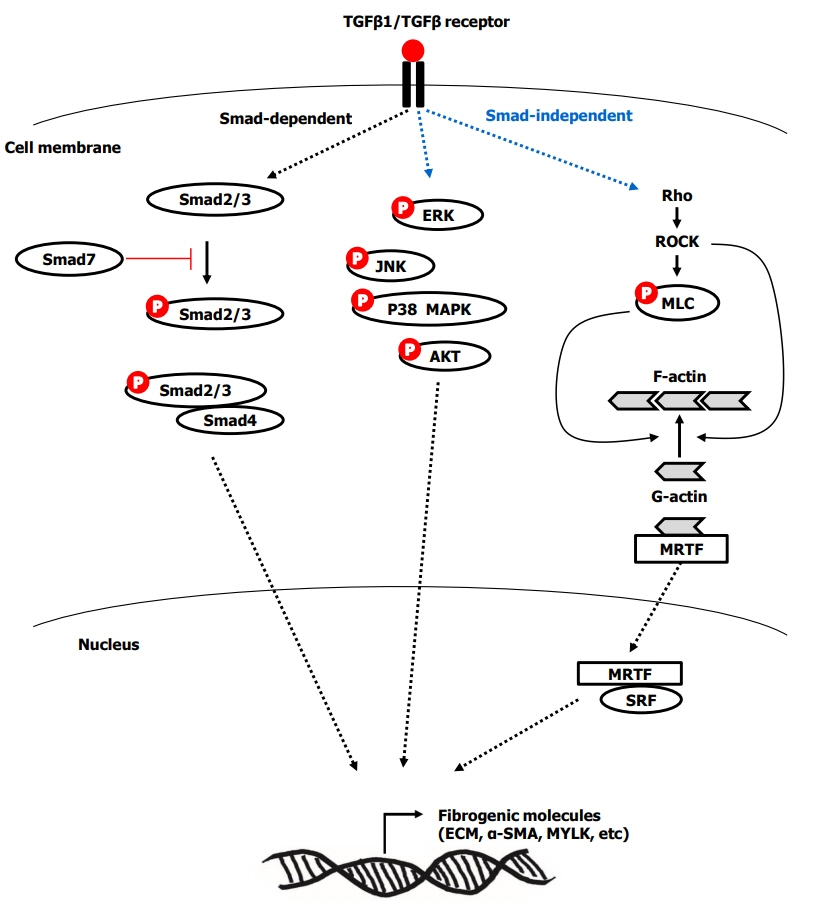
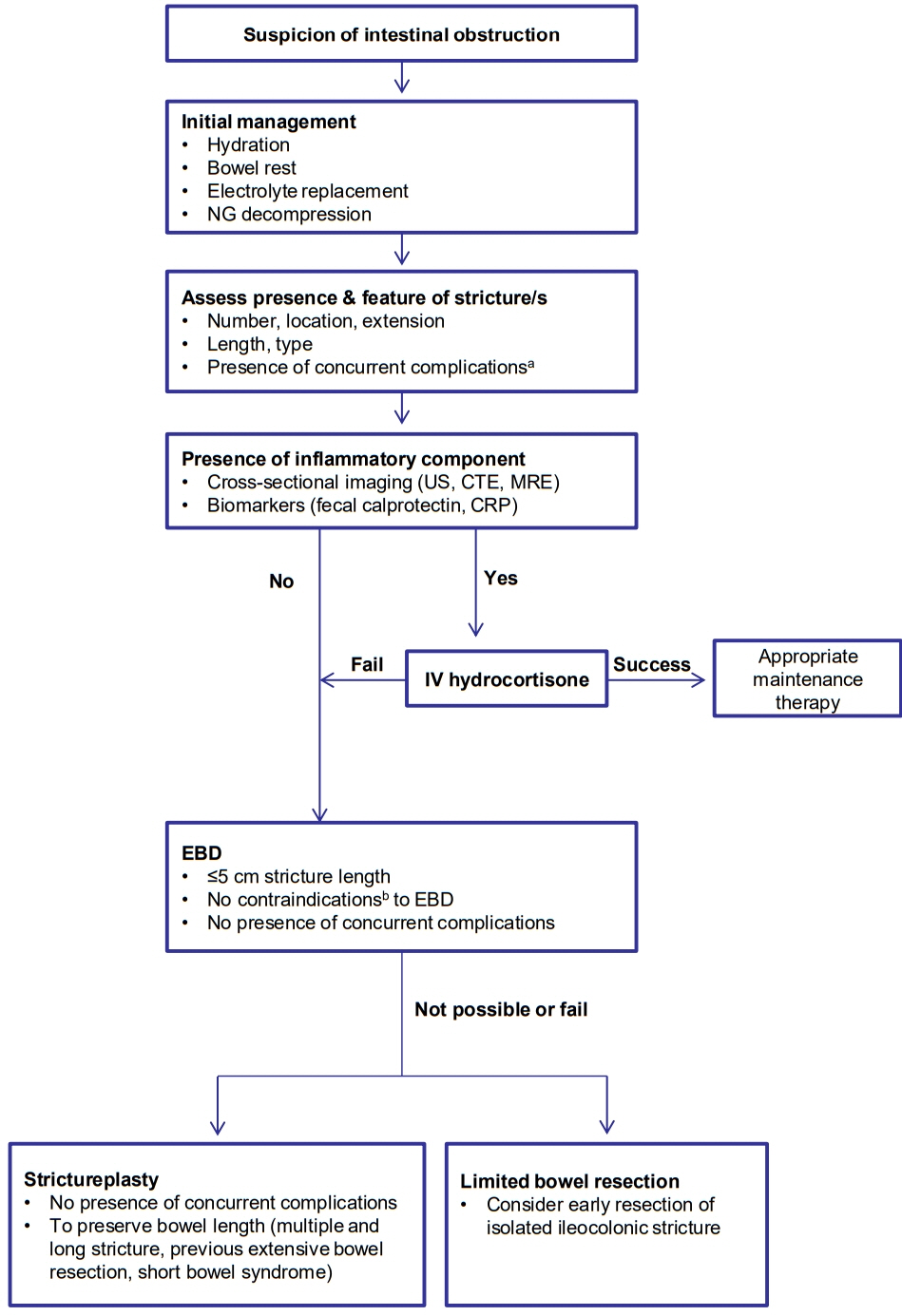
Fibrostenotic strictures in Crohn’s disease
- Affiliations
-
- 1Digestive Disease Center, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
- 2Department of Colorectal Surgery, Hepatology and Nutrition, Digestive Diseases and Surgery Institute, Cleveland Clinic Foundation, Cleveland, OH, USA
- 3Department of Gastroenterology, Hepatology and Nutrition, Digestive Diseases and Surgery Institute, Cleveland Clinic Foundation, Cleveland, OH, USA
- KMID: 2508564
- DOI: http://doi.org/10.5217/ir.2019.09148
Abstract
- The use of biologic agents including anti-tumor necrosis factor monoclonal antibodies followed by anti-integrins and anti-interleukins has drastically changed the treatment paradigm of Crohn’s disease (CD) by improving clinical symptoms and mucosal healing. However, up to 70% of CD patients still eventually undergo surgery mainly due to fibrostenotic strictures. There are no specific anti-fibrotic drugs yet. This review comprehensively addresses the mechanism, prediction, diagnosis and treatment of the fibrostenotic strictures in CD. We also introduce promising anti-fibrotic agents which may be available in the near future and summarize challenges in developing novel therapies to treat fibrostenotic strictures in CD.
Figure
Cited by 2 articles
-
Korean clinical practice guidelines on biologics for moderate to severe Crohn’s disease
Seong-Joon Koh, Sung Noh Hong, Soo-Kyung Park, Byong Duk Ye, Kyeong Ok Kim, Jeong Eun Shin, Yong Sik Yoon, Hong Sub Lee, Sung Hoon Jung, Miyoung Choi, Soo-Young Na, Chang Hwan Choi, Joo Sung Kim
Intest Res. 2023;21(1):43-60. doi: 10.5217/ir.2022.00029.Management of Crohn’s disease in Taiwan: consensus guideline of the Taiwan Society of Inflammatory Bowel Disease updated in 2023
Jia-Feng Wu, Hsu-Heng Yen, Horng-Yuan Wang, Ting-An Chang, Chung-Hsin Chang, Chen-Wang Chang, Te-Hsin Chao, Jen-Wei Chou, Yenn-Hwei Chou, Chiao-Hsiung Chuang, Wen-Hung Hsu, Tzu-Chi Hsu, Tien-Yu Huang, Tsung-I Hung, Puo-Hsien Le, Chun-Che Lin, Chun-Chi Lin, Ching-Pin Lin, Jen-Kou Lin, Wei-Chen Lin, Yen-Hsuan Ni, Ming-Jium Shieh, I-Lun Shih, Chia-Tung Shun, Tzung-Jiun Tsai, Cheng-Yi Wang, Meng-Tzu Weng, Jau-Min Wong, Deng-Chyang Wu, Shu-Chen Wei
Intest Res. 2024;22(3):250-285. doi: 10.5217/ir.2024.00060.
Reference
-
1. Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017; 152:340–350.
Article2. Rieder F. Managing intestinal fibrosis in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2018; 14:120–122.3. Lewis A, Nijhuis A, Mehta S, et al. Intestinal fibrosis in Crohn’s disease: role of microRNAs as fibrogenic modulators, serum biomarkers, and therapeutic targets. Inflamm Bowel Dis. 2015; 21:1141–1150.4. Rieder F, Bettenworth D, Ma C, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn’s disease. Aliment Pharmacol Ther. 2018; 48:347–357.
Article5. Shen B. Interventional IBD: the role of endoscopist in the multidisciplinary team management of IBD. Inflamm Bowel Dis. 2018; 24:298–309.
Article6. Munkholm P, Langholz E, Davidsen M, Binder V. Disease activity courses in a regional cohort of Crohn’s disease patients. Scand J Gastroenterol. 1995; 30:699–706.
Article7. Gklavas A, Dellaportas D, Papaconstantinou I. Risk factors for postoperative recurrence of Crohn’s disease with emphasis on surgical predictors. Ann Gastroenterol. 2017; 30:598–612.
Article8. Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sandborn WJ, Loftus EV Jr. Surgery in a population-based cohort of Crohn’s disease from Olmsted County, Minnesota (1970-2004). Am J Gastroenterol. 2012; 107:1693–1701.
Article9. Toh JW, Stewart P, Rickard MJ, Leong R, Wang N, Young CJ. Indications and surgical options for small bowel, large bowel and perianal Crohn’s disease. World J Gastroenterol. 2016; 22:8892–8904.
Article10. Buisson A, Chevaux JB, Allen PB, Bommelaer G, Peyrin-Biroulet L. Review article: the natural history of postoperative Crohn’s disease recurrence. Aliment Pharmacol Ther. 2012; 35:625–633.
Article11. Holvoet T, Devriese S, Castermans K, et al. Treatment of intestinal fibrosis in experimental inflammatory bowel disease by the pleiotropic actions of a local Rho kinase inhibitor. Gastroenterology. 2017; 153:1054–1067.
Article12. De Buck van Overstraeten A, Wolthuis A, D’Hoore A. Surgery for Crohn’s disease in the era of biologicals: a reduced need or delayed verdict? World J Gastroenterol. 2012; 18:3828–3832.
Article13. Chan WP, Mourad F, Leong RW. Crohn’s disease associated strictures. J Gastroenterol Hepatol. 2018; 33:998–1008.
Article14. Bettenworth D, Rieder F. Pathogenesis of intestinal fibrosis in inflammatory bowel disease and perspectives for therapeutic implication. Dig Dis. 2017; 35:25–31.
Article15. Latella G, Di Gregorio J, Flati V, Rieder F, Lawrance IC. Mechanisms of initiation and progression of intestinal fibrosis in IBD. Scand J Gastroenterol. 2015; 50:53–65.
Article16. Chen W, Lu C, Hirota C, Iacucci M, Ghosh S, Gui X. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in Crohn’s fibrostenosing bowel strictures: a semiquantitative analysis by using a novel histological grading scheme. J Crohns Colitis. 2017; 11:92–104.
Article17. Rieder F, Fiocchi C. Intestinal fibrosis in inflammatory bowel disease: current knowledge and future perspectives. J Crohns Colitis. 2008; 2:279–290.
Article18. Malgras B, Pautrat K, Dray X, et al. Multidisciplinary management of gastrointestinal fibrotic stenosis in Crohn’s disease. Dig Dis Sci. 2015; 60:1152–1168.
Article19. Rieder F, Latella G, Magro F, et al. European Crohn’s and Colitis Organisation topical review on prediction, diagnosis and management of fibrostenosing Crohn’s disease. J Crohns Colitis. 2016; 10:873–885.
Article20. Burke JP, Mulsow JJ, O’Keane C, Docherty NG, Watson RW, O’Connell PR. Fibrogenesis in Crohn’s disease. Am J Gastroenterol. 2007; 102:439–448.
Article21. Shepherd NA, Jass JR, Duval I, Moskowitz RL, Nicholls RJ, Morson BC. Restorative proctocolectomy with ileal reservoir: pathological and histochemical study of mucosal biopsy specimens. J Clin Pathol. 1987; 40:601–607.
Article22. Rieder F, Fiocchi C. Intestinal fibrosis in IBD: a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009; 6:228–235.
Article23. Lenti MV, Di Sabatino A. Intestinal fibrosis. Mol Aspects Med. 2019; 65:100–109.24. Valatas V, Filidou E, Drygiannakis I, Kolios G. Stromal and immune cells in gut fibrosis: the myofibroblast and the scarface. Ann Gastroenterol. 2017; 30:393–404.25. Graham MF, Willey A, Adams J, Diegelmann RF. Corticosteroids increase procollagen gene expression, synthesis, and secretion by human intestinal smooth muscle cells. Gastroenterology. 1995; 109:1454–1461.
Article26. Koo JB, Nam MO, Jung Y, et al. Anti-fibrogenic effect of PPAR-γ agonists in human intestinal myofibroblasts. BMC Gastroenterol. 2017; 17:73.
Article27. Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts: II. intestinal subepithelial myofibroblasts. Am J Physiol. 1999; 277–C183-C201.
Article28. Mifflin RC, Pinchuk IV, Saada JI, Powell DW. Intestinal myofibroblasts: targets for stem cell therapy. Am J Physiol Gastrointest Liver Physiol. 2011; 300–G684-G696.
Article29. Mostafa RM, Moustafa YM, Hamdy H. Interstitial cells of Cajal, the Maestro in health and disease. World J Gastroenterol. 2010; 16:3239–3248.
Article30. Otte JM, Rosenberg IM, Podolsky DK. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology. 2003; 124:1866–1878.
Article31. Zawahir S, Li G, Banerjee A, Shiu J, Blanchard TG, Okogbule-Wonodi AC. Inflammatory and immune activation in intestinal myofibroblasts is developmentally regulated. J Interferon Cytokine Res. 2015; 35:634–640.
Article32. Drygiannakis I, Valatas V, Sfakianaki O, et al. Proinflammatory cytokines induce crosstalk between colonic epithelial cells and subepithelial myofibroblasts: implication in intestinal fibrosis. J Crohns Colitis. 2013; 7:286–300.
Article33. Scharl M, Huber N, Lang S, Fürst A, Jehle E, Rogler G. Hallmarks of epithelial to mesenchymal transition are detectable in Crohn’s disease associated intestinal fibrosis. Clin Transl Med. 2015; 4:1.
Article34. Micallef L, Vedrenne N, Billet F, Coulomb B, Darby IA, Desmoulière A. The myofibroblast, multiple origins for major roles in normal and pathological tissue repair. Fibrogenesis Tissue Repair. 2012; 5(Suppl 1):S5.
Article35. Rieder F, Kessler SP, West GA, et al. Inflammation-induced endothelial-to-mesenchymal transition: a novel mechanism of intestinal fibrosis. Am J Pathol. 2011; 179:2660–2673.36. Lawrance IC, Rogler G, Bamias G, et al. Cellular and molecular mediators of intestinal fibrosis. J Crohns Colitis. 2017; 11:1491–1503.
Article37. Uehara H, Nakagawa T, Katsuno T, et al. Emergence of fibrocytes showing morphological changes in the inflamed colonic mucosa. Dig Dis Sci. 2010; 55:253–260.
Article38. Brittan M, Chance V, Elia G, et al. A regenerative role for bone marrow following experimental colitis: contribution to neovasculogenesis and myofibroblasts. Gastroenterology. 2005; 128:1984–1995.
Article39. Fiocchi C, Lund PK. Themes in fibrosis and gastrointestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2011; 300–G677-G683.
Article40. Biancheri P, Pender SL, Ammoscato F, et al. The role of interleukin 17 in Crohn’s disease-associated intestinal fibrosis. Fibrogenesis Tissue Repair. 2013; 6:13.
Article41. Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006; 12:99–106.
Article42. Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004; 4:583–594.
Article43. Medina C, Santos-Martinez MJ, Santana A, et al. Transforming growth factor-beta type 1 receptor (ALK5) and Smad proteins mediate TIMP-1 and collagen synthesis in experimental intestinal fibrosis. J Pathol. 2011; 224:461–472.
Article44. Sanders YY, Cui Z, Le Saux CJ, et al. SMAD-independent down-regulation of caveolin-1 by TGF-beta: effects on proliferation and survival of myofibroblasts. PLoS One. 2015; 10:e0116995.45. Lawrance IC, Maxwell L, Doe W. Altered response of intestinal mucosal fibroblasts to profibrogenic cytokines in inflammatory bowel disease. Inflamm Bowel Dis. 2001; 7:226–236.
Article46. Leeb SN, Vogl D, Falk W, Schölmerich J, Rogler G, Gelbmann CM. Regulation of migration of human colonic myofibroblasts. Growth Factors. 2002; 20:81–91.
Article47. Lin X, Wen J, Liu R, Gao W, Qu B, Yu M. Nintedanib inhibits TGF-beta-induced myofibroblast transdifferentiation in human Tenon’s fibroblasts. Mol Vis. 2018; 24:789–800.48. Latella G, Sferra R, Speca S, Vetuschi A, Gaudio E. Can we prevent, reduce or reverse intestinal fibrosis in IBD? Eur Rev Med Pharmacol Sci. 2013; 17:1283–1304.49. Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003; 425:577–584.
Article50. Mu Y, Gudey SK, Landström M. Non-Smad signaling pathways. Cell Tissue Res. 2012; 347:11–20.
Article51. Johnson LA, Rodansky ES, Haak AJ, Larsen SD, Neubig RR, Higgins PD. Novel Rho/MRTF/SRF inhibitors block matrix-stiffness and TGF-beta-induced fibrogenesis in human colonic myofibroblasts. Inflamm Bowel Dis. 2014; 20:154–165.
Article52. Small EM. The actin-MRTF-SRF gene regulatory axis and myofibroblast differentiation. J Cardiovasc Transl Res. 2012; 5:794–804.
Article53. Choi YJ, Koo JB, Kim HY, et al. Umbilical cord/placenta-derived mesenchymal stem cells inhibit fibrogenic activation in human intestinal myofibroblasts via inhibition of myocardin-related transcription factor A. Stem Cell Res Ther. 2019; 10:291.
Article54. Hinz B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep. 2009; 11:120–126.
Article55. Latella G, Rogler G, Bamias G, et al. Results of the 4th Scientific Workshop of the ECCO (I): pathophysiology of intestinal fibrosis in IBD. J Crohns Colitis. 2014; 8:1147–1165.
Article56. Fichtner-Feigl S, Fuss IJ, Young CA, et al. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6- trinitrobenzene sulfonic acid colitis. J Immunol. 2007; 178:5859–5870.
Article57. Fichtner-Feigl S, Young CA, Kitani A, Geissler EK, Schlitt HJ, Strober W. IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology. 2008; 135:2003–2013.58. Scheibe K, Kersten C, Schmied A, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology. 2019; 156:1082–1097.
Article59. Mao R, Rieder F. Cooling down the hot potato: anti-interleukin 36 therapy prevents and treats experimental intestinal fibrosis. Gastroenterology. 2019; 156:871–873.
Article60. Scheibe K, Backert I, Wirtz S, et al. IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut. 2017; 66:823–838.
Article61. Shih DQ, Zheng L, Zhang X, et al. Inhibition of a novel fibrogenic factor Tl1a reverses established colonic fibrosis. Mucosal Immunol. 2014; 7:1492–1503.
Article62. Shih DQ, Barrett R, Zhang X, et al. Constitutive TL1A (TNFSF15) expression on lymphoid or myeloid cells leads to mild intestinal inflammation and fibrosis. PLoS One. 2011; 6:e16090.
Article63. Barrett R, Zhang X, Koon HW, et al. Constitutive TL1A expression under colitogenic conditions modulates the severity and location of gut mucosal inflammation and induces fibrostenosis. Am J Pathol. 2012; 180:636–649.
Article64. Li H, Song J, Niu G, et al. TL1A blocking ameliorates intestinal fibrosis in the T cell transfer model of chronic colitis in mice. Pathol Res Pract. 2018; 214:217–227.
Article65. Okamoto R, Watanabe M. Role of epithelial cells in the pathogenesis and treatment of inflammatory bowel disease. J Gastroenterol. 2016; 51:11–21.
Article66. Rieder F. The gut microbiome in intestinal fibrosis: environmental protector or provocateur? Sci Transl Med. 2013; 5:190–ps10.
Article67. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010; 140:805–820.
Article68. Rieder F, Bhilocha S, Schirbel A, et al. Activation of Toll-like receptor (TLR) 5 induces a pro-fibrogenic phenotype on human intestinal myofibroblasts (HIF): a novel pathway mediated by caspase 1. Gastroenterology. 2011; 140:S–114.69. Hasan UA, Trinchieri G, Vlach J. Toll-like receptor signaling stimulates cell cycle entry and progression in fibroblasts. J Biol Chem. 2005; 280:20620–20627.
Article70. Imai J, Kitamoto S, Sugihara K, et al. Flagellin-mediated activation of IL-33-ST2 signaling by a pathobiont promotes intestinal fibrosis. Mucosal Immunol. 2019; 12:632–643.
Article71. Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004; 113:1296–1306.
Article72. Targan SR, Landers CJ, Yang H, et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005; 128:2020–2028.
Article73. Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001; 411:599–603.
Article74. Economou M, Trikalinos TA, Loizou KT, Tsianos EV, Ioannidis JP. Differential effects of NOD2 variants on Crohn’s disease risk and phenotype in diverse populations: a metaanalysis. Am J Gastroenterol. 2004; 99:2393–2404.
Article75. Mourelle M, Salas A, Guarner F, Crespo E, García-Lafuente A, Malagelada JR. Stimulation of transforming growth factor beta1 by enteric bacteria in the pathogenesis of rat intestinal fibrosis. Gastroenterology. 1998; 114:519–526.
Article76. Pucilowska JB, Williams KL, Lund PK. Fibrogenesis. IV. Fibrosis and inflammatory bowel disease: cellular mediators and animal models. Am J Physiol Gastrointest Liver Physiol. 2000; 279–G653-G659.
Article77. Rieder F, Kessler S, Sans M, Fiocchi C. Animal models of intestinal fibrosis: new tools for the understanding of pathogenesis and therapy of human disease. Am J Physiol Gastrointest Liver Physiol. 2012; 303–G786-G801.
Article78. Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008; 47:1394–1400.
Article79. Johnson LA, Rodansky ES, Sauder KL, et al. Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts. Inflamm Bowel Dis. 2013; 19:891–903.
Article80. Johnson LA, Luke A, Sauder K, Moons DS, Horowitz JC, Higgins PD. Intestinal fibrosis is reduced by early elimination of inflammation in a mouse model of IBD: impact of a “top-down” approach to intestinal fibrosis in mice. Inflamm Bowel Dis. 2012; 18:460–471.
Article81. Peyrin-Biroulet L, Chamaillard M, Gonzalez F, et al. Mesenteric fat in Crohn’s disease: a pathogenetic hallmark or an innocent bystander? Gut. 2007; 56:577–583.82. Sheehan AL, Warren BF, Gear MW, Shepherd NA. Fat-wrapping in Crohn’s disease: pathological basis and relevance to surgical practice. Br J Surg. 1992; 79:955–958.
Article83. Kredel LI, Batra A, Stroh T, et al. Adipokines from local fat cells shape the macrophage compartment of the creeping fat in Crohn’s disease. Gut. 2013; 62:852–862.
Article84. Lech M, Anders HJ. Macrophages and fibrosis: how resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 2013; 1832:989–997.
Article85. Mao R, Kurada S, Gordon IO, et al. The mesenteric fat and intestinal muscle interface: creeping fat influencing stricture formation in Crohn’s disease. Inflamm Bowel Dis. 2019; 25:421–426.
Article86. Rieder F, Doyon G, Ouyang Z, West G, Fiocchi C. 573 Adipocyte and preadipocyte derived-mediators induce a PRO-fibrogenic phenotype in human intestinal mesenchymal cells: a novel link between fat and intestinal fibrosis. Gastroenterology. 2014; 146:S–106.
Article87. Di Sabatino A, Jackson CL, Pickard KM, et al. Transforming growth factor beta signalling and matrix metalloproteinases in the mucosa overlying Crohn’s disease strictures. Gut. 2009; 58:777–789.
Article88. Monteleone G, Pallone F, MacDonald TT. Smad7 in TGF-beta-mediated negative regulation of gut inflammation. Trends Immunol. 2004; 25:513–517.89. Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001; 108:601–609.
Article90. Biancheri P, Giuffrida P, Docena GH, MacDonald TT, Corazza GR, Di Sabatino A. The role of transforming growth factor (TGF)-beta in modulating the immune response and fibrogenesis in the gut. Cytokine Growth Factor Rev. 2014; 25:45–55.
Article91. Li C, Kuemmerle JF. Tu1772: epigenetic silencing of Smad7 contributes to fibrosis in stricturing Crohn’s disease. Gastroenterology. 2018; 154:S–1015.92. Monteleone G, Mann J, Monteleone I, et al. A failure of transforming growth factor-beta1 negative regulation maintains sustained NF-kappaB activation in gut inflammation. J Biol Chem. 2004; 279:3925–3932.
Article93. Kennedy BW. Mongersen: an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N Engl J Med. 2015; 372:2461.94. Zorzi F, Calabrese E, Monteleone I, et al. A phase 1 open-label trial shows that smad7 antisense oligonucleotide (GED0301) does not increase the risk of small bowel strictures in Crohn’s disease. Aliment Pharmacol Ther. 2012; 36:850–857.
Article95. Nijhuis A, Biancheri P, Lewis A, et al. In Crohn’s disease fibrosis-reduced expression of the miR-29 family enhances collagen expression in intestinal fibroblasts. Clin Sci (Lond). 2014; 127:341–350.
Article96. Lewis A, Mehta S, Hanna LN, et al. Low serum levels of microRNA-19 are associated with a stricturing Crohn’s disease phenotype. Inflamm Bowel Dis. 2015; 21:1926–1934.
Article97. Tarrant KM, Barclay ML, Frampton CM, Gearry RB. Perianal disease predict changes in Crohn’s disease phenotype: results of a population-based study of inflammatory bowel disease phenotype. Am J Gastroenterol. 2008; 103:3082–3093.
Article98. Romberg-Camps MJ, Dagnelie PC, Kester AD, et al. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol. 2009; 104:371–383.
Article99. Lakatos PL, Czegledi Z, Szamosi T, et al. Perianal disease, small bowel disease, smoking, prior steroid or early azathioprine/biological therapy are predictors of disease behavior change in patients with Crohn’s disease. World J Gastroenterol. 2009; 15:3504–3510.
Article100. Rieder F, Lawrance IC, Leite A, Sans M. Predictors of fibrostenotic Crohn’s disease. Inflamm Bowel Dis. 2011; 17:2000–2007.
Article101. Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV Jr. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010; 139:1147–1155.
Article102. Louis E, Michel V, Hugot JP, et al. Early development of stricturing or penetrating pattern in Crohn’s disease is influenced by disease location, number of flares, and smoking but not by NOD2/CARD15 genotype. Gut. 2003; 52:552–557.
Article103. Alvarez-Lobos M, Arostegui JI, Sans M, et al. Crohn’s disease patients carrying Nod2/CARD15 gene variants have an increased and early need for first surgery due to stricturing disease and higher rate of surgical recurrence. Ann Surg. 2005; 242:693–700.
Article104. Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011; 9:356–368.
Article105. Adler J, Rangwalla SC, Dwamena BA, Higgins PD. The prognostic power of the NOD2 genotype for complicated Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2011; 106:699–712.
Article106. Brant SR, Picco MF, Achkar JP, et al. Defining complex contributions of NOD2/CARD15 gene mutations, age at onset, and tobacco use on Crohn’s disease phenotypes. Inflamm Bowel Dis. 2003; 9:281–289.
Article107. Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013; 62:1072–1084.
Article108. Lakatos PL, Papp M, Rieder F. Serologic antiglycan antibodies in inflammatory bowel disease. Am J Gastroenterol. 2011; 106:406–412.
Article109. Chen Y, Ge W, Xu L, et al. miR-200b is involved in intestinal fibrosis of Crohn’s disease. Int J Mol Med. 2012; 29:601–606.
Article110. Giuffrida P, Pinzani M, Corazza GR, Di Sabatino A. Biomarkers of intestinal fibrosis: one step towards clinical trials for stricturing inflammatory bowel disease. United European Gastroenterol J. 2016; 4:523–530.
Article111. Allez M, Lemann M, Bonnet J, Cattan P, Jian R, Modigliani R. Long term outcome of patients with active Crohn’s disease exhibiting extensive and deep ulcerations at colonoscopy. Am J Gastroenterol. 2002; 97:947–953.
Article112. Bettenworth D, Bokemeyer A, Baker M, et al. Assessment of Crohn’s disease-associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review. Gut. 2019; 68:1115–1126.
Article113. Adler J, Punglia DR, Dillman JR, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn’s disease. Inflamm Bowel Dis. 2012; 18:849–856.
Article114. Maconi G, Carsana L, Fociani P, et al. Small bowel stenosis in Crohn’s disease: clinical, biochemical and ultrasonographic evaluation of histological features. Aliment Pharmacol Ther. 2003; 18:749–756.
Article115. Pallotta N, Vincoli G, Montesani C, et al. Small intestine contrast ultrasonography (SICUS) for the detection of small bowel complications in Crohn’s disease: a prospective comparative study versus intraoperative findings. Inflamm Bowel Dis. 2012; 18:74–84.
Article116. Vogel J, da Luz Moreira A, Baker M, et al. CT enterography for Crohn’s disease: accurate preoperative diagnostic imaging. Dis Colon Rectum. 2007; 50:1761–1769.
Article117. Kumar S, Hakim A, Alexakis C, et al. Small intestinal contrast ultrasonography for the detection of small bowel complications in Crohn’s disease: correlation with intraoperative findings and magnetic resonance enterography. J Gastroenterol Hepatol. 2015; 30:86–91.
Article118. Pous-Serrano S, Frasson M, Palasí Giménez R, et al. Accuracy of magnetic resonance enterography in the preoperative assessment of patients with Crohn’s disease of the small bowel. Colorectal Dis. 2017; 19–O126-O133.
Article119. Sinha R, Murphy P, Sanders S, et al. Diagnostic accuracy of high-resolution MR enterography in Crohn’s disease: comparison with surgical and pathological specimen. Clin Radiol. 2013; 68:917–927.
Article120. Takenaka K, Ohtsuka K, Kitazume Y, et al. Comparison of magnetic resonance and balloon enteroscopic examination of the small intestine in patients with Crohn’s disease. Gastroenterology. 2014; 147:334–342.
Article121. Castiglione F, Mainenti PP, De Palma GD, et al. Noninvasive diagnosis of small bowel Crohn’s disease: direct comparison of bowel sonography and magnetic resonance enterography. Inflamm Bowel Dis. 2013; 19:991–998.122. Orlando S, Fraquelli M, Coletta M, et al. Ultrasound elasticity imaging predicts therapeutic outcomes of patients with Crohn’s disease treated with anti-tumour necrosis factor antibodies. J Crohns Colitis. 2018; 12:63–70.
Article123. Liu YB, Liang CH, Zhang ZL, et al. Crohn disease of small bowel: multidetector row CT with CT enteroclysis, dynamic contrast enhancement, CT angiography, and 3D imaging. Abdom Imaging. 2006; 31:668–674.
Article124. Rimola J, Planell N, Rodríguez S, et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015; 110:432–440.
Article125. Gramlich T, Petras RE. Pathology of inflammatory bowel disease. Semin Pediatr Surg. 2007; 16:154–163.
Article126. Brahme F, Lindström C. A comparative radiographic and pathological study of intestinal vaso-architecture in Crohn’s disease and in ulcerative colitis. Gut. 1970; 11:928–940.
Article127. Li XH, Mao R, Huang SY, et al. Characterization of degree of intestinal fibrosis in patients with Crohn disease by using magnetization transfer MR imaging. Radiology. 2018; 287:494–503.
Article128. Allocca M, Fiorino G, Bonifacio C, Peyrin-Biroulet L, Danese S. Noninvasive multimodal methods to differentiate inflamed vs fibrotic strictures in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2019; 17:2397–2415.
Article129. Choi SH, Kim KW, Lee JY, Kim KJ, Park SH. Diffusion-weighted magnetic resonance enterography for evaluating bowel inflammation in Crohn’s disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2016; 22:669–679.
Article130. Tielbeek JA, Ziech ML, Li Z, et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol. 2014; 24:619–629.
Article131. Adler J, Swanson SD, Schmiedlin-Ren P, et al. Magnetization transfer helps detect intestinal fibrosis in an animal model of Crohn disease. Radiology. 2011; 259:127–135.
Article132. Moy MP, Sauk J, Gee MS. The role of MR enterography in assessing Crohn’s disease activity and treatment response. Gastroenterol Res Pract. 2016; 2016:8168695.
Article133. Gordon IO, Bettenworth D, Bokemeyer A, et al. Histopathology scoring systems of stenosis associated with small bowel Crohn’s disease: a systematic review. Gastroenterology. 2020; 158:137–150.
Article134. Benitez JM, Meuwis MA, Reenaers C, van Kemseke C, Meunier P, Louis E. Role of endoscopy, cross-sectional imaging and biomarkers in Crohn’s disease monitoring. Gut. 2013; 62:1806–1816.
Article135. Pariente B, Cosnes J, Danese S, et al. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm Bowel Dis. 2011; 17:1415–1422.
Article136. Yaffe BH, Korelitz BI. Prognosis for nonoperative management of small-bowel obstruction in Crohn’s disease. J Clin Gastroenterol. 1983; 5:211–215.
Article137. Gordon M. 5-Aminosalicylates to maintain remission in Crohn’s disease: interpreting conflicting systematic review evidence. World J Gastrointest Pharmacol Ther. 2017; 8:99–102.
Article138. de Souza GS, Vidigal FM, Chebli LA, et al. Effect of azathioprine or mesalazine therapy on incidence of re-hospitalization in sub-occlusive ileocecal Crohn’s disease patients. Med Sci Monit. 2013; 19:716–722.
Article139. Reinisch W, Angelberger S, Petritsch W, et al. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in patients with Crohn’s disease with endoscopic recurrence: efficacy and safety results of a randomised, double-blind, double-dummy, multicentre trial. Gut. 2010; 59:752–759.
Article140. Ardizzone S, Maconi G, Sampietro GM, et al. Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn’s disease. Gastroenterology. 2004; 127:730–740.
Article141. Speca S, Rousseaux C, Dubuquoy C, et al. Novel PPARγ modulator GED-0507-34 Levo ameliorates inflammation-driven intestinal fibrosis. Inflamm Bowel Dis. 2016; 22:279–292.
Article142. Szabò H, Fiorino G, Spinelli A, et al. Review article: anti-fibrotic agents for the treatment of Crohn’s disease: lessons learnt from other diseases. Aliment Pharmacol Ther. 2010; 31:189–201.143. Videla S, Vilaseca J, Medina C, et al. Selective inhibition of phosphodiesterase-4 ameliorates chronic colitis and prevents intestinal fibrosis. J Pharmacol Exp Ther. 2006; 316:940–945.
Article144. Peyrin-Biroulet L, Deltenre P, Ardizzone S, et al. Azathioprine and 6-mercaptopurine for the prevention of postoperative recurrence in Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2009; 104:2089–2096.
Article145. Casanova MJ, Chaparro M, García-Sánchez V, et al. Evolution after anti-TNF discontinuation in patients with inflammatory bowel disease: a multicenter long-term follow-up study. Am J Gastroenterol. 2017; 112:120–131.146. Lichtenstein GR, Olson A, Travers S, et al. Factors associated with the development of intestinal strictures or obstructions147. Bouhnik Y, Carbonnel F, Laharie D, et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut. 2018; 67:53–60.
Article147. Bouhnik Y, Carbonnel F, Laharie D, et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut. 2018; 67:53–60.
Article148. Fields AC, Melnitchouk N. Medical prophylaxis of post-surgical Crohn’s disease recurrence: towards timely anti-TNF therapy. Dig Dis Sci. 2019; 64:7–8.
Article149. Nguyen GC, Loftus EV Jr, Hirano I, et al. American Gastroenterological Association Institute guideline on the management of Crohn’s disease after surgical resection. Gastroenterology. 2017; 152:271–275.
Article150. Ding NS, Yip WM, Choi CH, et al. Endoscopic dilatation of Crohn’s anastomotic strictures is effective in the long term, and escalation of medical therapy improves outcomes in the biologic era. J Crohns Colitis. 2016; 10:1172–1178.
Article151. Kopylov U, Verstockt B, Biedermann L, et al. Effectiveness and safety of vedolizumab in anti-TNF-naïve patients with inflammatory bowel disease-a multicenter retrospective European study. Inflamm Bowel Dis. 2018; 24:2442–2451.
Article152. Dulai PS, Singh S, Jiang X, et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: results from the US VICTORY consortium. Am J Gastroenterol. 2016; 111:1147–1155.
Article153. Ma C, Fedorak RN, Kaplan GG, et al. Clinical, endoscopic and radiographic outcomes with ustekinumab in medically-refractory Crohn’s disease: real world experience from a multicenter cohort. Aliment Pharmacol Ther. 2017; 45:1232–1243.
Article154. Hirai F, Andoh A, Ueno F, et al. Efficacy of endoscopic balloon dilation for small bowel strictures in patients with Crohn’s disease: a nationwide, multi-centre, open-label, prospective cohort study. J Crohns Colitis. 2018; 12:394–401.
Article155. Bettenworth D, Gustavsson A, Atreja A, et al. A pooled analysis of efficacy, safety, and long-term outcome of endoscopic balloon dilation therapy for patients with stricturing Crohn’s disease. Inflamm Bowel Dis. 2017; 23:133–142.
Article156. Shen B, Kochhar G, Navaneethan U, et al. Role of interventional inflammatory bowel disease in the era of biologic therapy: a position statement from the Global Interventional IBD Group. Gastrointest Endosc. 2019; 89:215–237.
Article157. Chen M, Shen B. Comparable short- and long-term outcomes of colonoscopic balloon dilation of Crohn’s disease and benign non-Crohn’s disease strictures. Inflamm Bowel Dis. 2014; 20:1739–1746.
Article158. Lian L, Stocchi L, Remzi FH, Shen B. Comparison of endoscopic dilation vs surgery for anastomotic stricture in patients with Crohn’s disease following ileocolonic resection. Clin Gastroenterol Hepatol. 2017; 15:1226–1231.
Article159. Bettenworth D, Lopez R, Hindryckx P, Levesque BG, Rieder F. Heterogeneity in endoscopic treatment of Crohn’s disease-associated strictures: an international inflammatory bowel disease specialist survey. J Gastroenterol. 2016; 51:939–948.
Article160. Chen M, Shen B. Endoscopic therapy in Crohn’s disease: principle, preparation, and technique. Inflamm Bowel Dis. 2015; 21:2222–2240.161. Loras Alastruey C, Andújar Murcia X, Esteve Comas M. The role of stents in the treatment of Crohn’s disease strictures. Endosc Int Open. 2016; 4:E301–E308.
Article162. Levine RA, Wasvary H, Kadro O. Endoprosthetic management of refractory ileocolonic anastomotic strictures after resection for Crohn’s disease: report of nine-year follow-up and review of the literature. Inflamm Bowel Dis. 2012; 18:506–512.
Article163. Campos C, Perrey A, Lambert C, et al. Medical therapies for stricturing Crohn’s disease: efficacy and cross-sectional imaging predictors of therapeutic failure. Dig Dis Sci. 2017; 62:1628–1636.
Article164. Morar PS, Faiz O, Warusavitarne J, et al. Systematic review with meta-analysis: endoscopic balloon dilatation for Crohn’s disease strictures. Aliment Pharmacol Ther. 2015; 42:1137–1148.
Article165. Golovics PA, Lakatos L, Nagy A, et al. Is early limited surgery associated with a more benign disease course in Crohn’s disease? World J Gastroenterol. 2013; 19:7701–7710.
Article166. Kulungowski AM, Acker SN, Hoffenberg EJ, Neigut D, Partrick DA. Initial operative treatment of isolated ileal Crohn’s disease in adolescents. Am J Surg. 2015; 210:141–145.
Article167. Latella G, Cocco A, Angelucci E, et al. Clinical course of Crohn’s disease first diagnosed at surgery for acute abdomen. Dig Liver Dis. 2009; 41:269–276.
Article168. Lee CH, Rieder F, Holubar SD. Duodenojejunal bypass and strictureplasty for diffuse small bowel Crohn’s disease with a step-by-step visual guide. Crohns Colitis 360. 2019; 1–otz002.
Article169. Ambe R, Campbell L, Cagir B. A comprehensive review of strictureplasty techniques in Crohn’s disease: types, indications, comparisons, and safety. J Gastrointest Surg. 2012; 16:209–217.
Article170. Bettenworth D, Rieder F. Reversibility of stricturing Crohn’s disease: fact or fiction? Inflamm Bowel Dis. 2016; 22:241–247.171. Maconi G, Sampietro GM, Cristaldi M, et al. Preoperative characteristics and postoperative behavior of bowel wall on risk of recurrence after conservative surgery in Crohn’s disease: a prospective study. Ann Surg. 2001; 233:345–352.
Article172. Yamamoto T, Fazio VW, Tekkis PP. Safety and efficacy of strictureplasty for Crohn’s disease: a systematic review and meta-analysis. Dis Colon Rectum. 2007; 50:1968–1986.
Article173. Oku H, Shimizu T, Kawabata T, et al. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol. 2008; 590:400–408.
Article174. Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011; 377:1760–1769.
Article175. Li G, Ren J, Hu Q, et al. Oral pirfenidone protects against fibrosis by inhibiting fibroblast proliferation and TGF-beta signaling in a murine colitis model. Biochem Pharmacol. 2016; 117:57–67.
Article176. Sun YW, Zhang YY, Ke XJ, Wu XJ, Chen ZF, Chi P. Pirfenidone prevents radiation-induced intestinal fibrosis in rats by inhibiting fibroblast proliferation and differentiation and suppressing the TGF-beta1/Smad/CTGF signaling pathway. Eur J Pharmacol. 2018; 822:199–206.
Article177. Meier R, Lutz C, Cosín-Roger J, et al. Decreased fibrogenesis after treatment with pirfenidone in a newly developed mouse model of intestinal fibrosis. Inflamm Bowel Dis. 2016; 22:569–582.
Article178. Kadir SI, Wenzel Kragstrup T, Dige A, Kok Jensen S, Dahlerup JF, Kelsen J. Pirfenidone inhibits the proliferation of fibroblasts from patients with active Crohn’s disease. Scand J Gastroenterol. 2016; 51:1321–1325.
Article179. Huang J, Beyer C, Palumbo-Zerr K, et al. Nintedanib inhibits fibroblast activation and ameliorates fibrosis in preclinical models of systemic sclerosis. Ann Rheum Dis. 2016; 75:883–890.
Article180. Maher TM, Strek ME. Antifibrotic therapy for idiopathic pulmonary fibrosis: time to treat. Respir Res. 2019; 20:205.
Article181. Rieder F. ROCKing the field of intestinal fibrosis or between a ROCK and a hard place? Gastroenterology. 2017; 153:895–897.
Article182. Bettenworth D, Rieder F. Medical therapy of stricturing Crohn’s disease: what the gut can learn from other organs: a systematic review. Fibrogenesis Tissue Repair. 2014; 7:5.183. Danese S, Bonovas S, Lopez A, et al. Identification of endpoints for development of antifibrosis drugs for treatment of Crohn’s disease. Gastroenterology. 2018; 155:76–87.
Article184. Pariente B, Hu S, Bettenworth D, et al. Treatments for Crohn’s disease-associated bowel damage: a systematic review. Clin Gastroenterol Hepatol. 2019; 17:847–856.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic Balloon Dilation for Crohn’s Disease-Associated Strictures
- Current status of endoscopic balloon dilation for Crohn's disease
- Update of endoscopic management of Crohn’s disease strictures
- Differentiation of fibrotic and inflammatory component of Crohn’s disease-associated strictures
- New Aspects of surgical therapy of recurrent Crohn's disease