Intest Res.
2020 Apr;18(2):144-150. 10.5217/ir.2020.00015.
Differentiation of fibrotic and inflammatory component of Crohn’s disease-associated strictures
- Affiliations
-
- 1Department of Radiology, Hospital Clinic Barcelona, Barcelona, Spain
- 2Centro de Investigación Biomédica en Red Enfermedades Hepáticas y Digestivas (CIBER-EHD), Spain
- 3Department of Radiology, University Hospital Sant’Orsola Malpighi, Bologna, Italy
- KMID: 2501380
- DOI: http://doi.org/10.5217/ir.2020.00015
Abstract
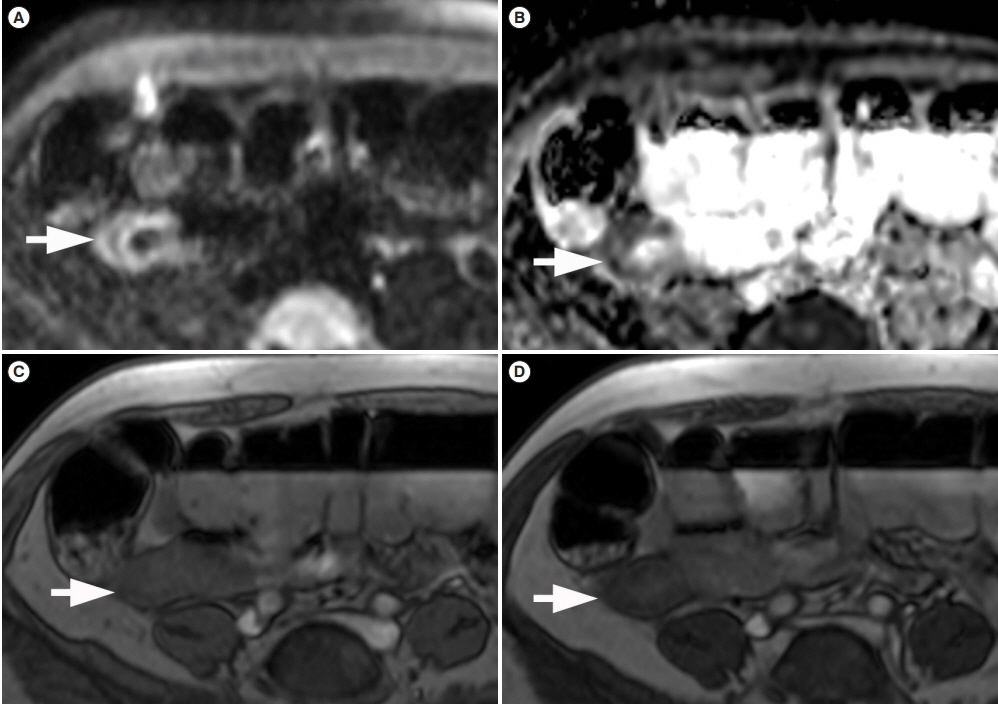
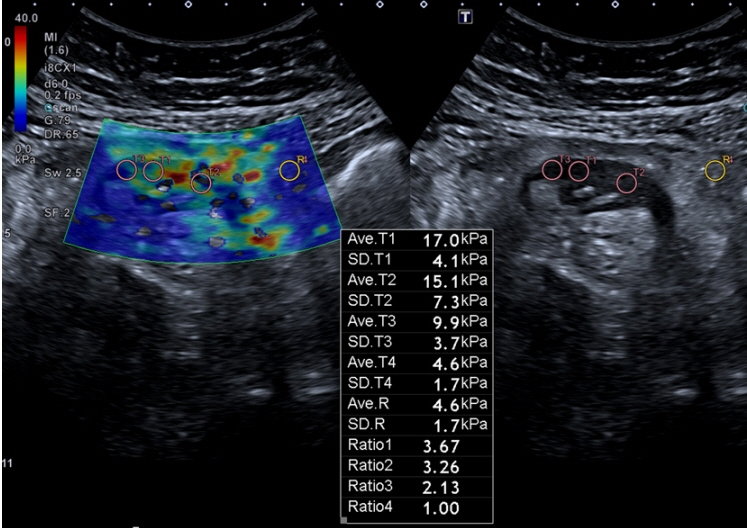
- Patients with Crohn’s disease (CD) commonly develop bowel strictures, which may contain various degrees of inflammation and fibrosis. While predominantly inflammatory strictures may benefit from a medical anti-inflammatory treatment approach, fibrotic strictures would require endoscopic balloon dilation or surgery. Cross-sectional imaging surpasses endoscopy for characterization of stenotic segments and potentially may contribute to the optimal clinical management of these patients. This short review aims to discuss the potentialities and limitations of cross-sectional imaging techniques for assessing bowel fibrosis in patients with CD.
Keyword
Figure
Cited by 1 articles
-
Crohn’s disease at radiological imaging: focus on techniques and intestinal tract
Giuseppe Cicero, Silvio Mazziotti
Intest Res. 2021;19(4):365-378. doi: 10.5217/ir.2020.00097.
Reference
-
1. Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013; 62:1072–1084.
Article2. Rieder F, Latella G, Magro F, et al. European Crohn’s and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn’s Disease. J Crohns Colitis. 2016; 10:873–885.
Article3. Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016. Part 1: diagnosis and medical management. J Crohns Colitis. 2017; 11:3–25.
Article4. Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002; 8:244–250.
Article5. Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001; 49:777–782.
Article6. Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017; 152:340–350.
Article7. Chiorean MV, Sandrasegaran K, Saxena R, Maglinte DD, Nakeeb A, Johnson CS. Correlation of CT enteroclysis with surgical pathology in Crohn’s disease. Am J Gastroenterol. 2007; 102:2541–2550.
Article8. Adler J, Punglia DR, Dillman JR, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn’s disease. Inflamm Bowel Dis. 2012; 18:849–856.
Article9. Zappa M, Stefanescu C, Cazals-Hatem D, et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn’s disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis. 2011; 17:984–993.
Article10. Rimola J, Planell N, Rodríguez S, et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015; 110:432–440.
Article11. Punwani S, Rodriguez-Justo M, Bainbridge A, et al. Mural inflammation in Crohn disease: location-matched histologic validation of MR imaging features. Radiology. 2009; 252:712. 720.
Article12. Stidham RW, Xu J, Johnson LA, et al. Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with Crohn’s disease. Gastroenterology. 2011; 141:819–826.
Article13. Tielbeek JA, Ziech ML, Li Z, et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol. 2014; 24:619–629.
Article14. Li XH, Mao R, Huang SY, et al. Characterization of degree of intestinal fibrosis in patients with Crohn disease by using magnetization transfer MR imaging. Radiology. 2018; 287:494–503.
Article15. Wagner M, Ko HM, Chatterji M, et al. Magnetic resonance imaging predicts histopathological composition of ileal Crohn’s disease. J Crohns Colitis. 2018; 12:718–729.
Article16. Rieder F, Bettenworth D, Ma C, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn’s disease. Aliment Pharmacol Ther. 2018; 48:347–357.
Article17. Jairath V, Levesque BG, Vande Casteele N, et al. Evolving concepts in phases I and II drug development for Crohn’s disease. J Crohns Colitis. 2017; 11:246–255.
Article18. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019; 13:144–164.
Article19. Bruining DH, Zimmermann EM, Loftus EV Jr, et al. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Gastroenterology. 2018; 154:1172–1194.
Article20. Bettenworth D, Bokemeyer A, Baker M, et al. Assessment of Crohn’s disease-associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review. Gut. 2019; 68:1115–1126.
Article21. Wilkens R, Hagemann-Madsen RH, Peters DA, et al. Validity of contrast-enhanced ultrasonography and dynamic contrast-enhanced MR enterography in the assessment of transmural activity and fibrosis in Crohn’s disease. J Crohns Colitis. 2018; 12:48–56.
Article22. Dillman JR, Stidham RW, Higgins PD, et al. Ultrasound shear wave elastography helps discriminate low-grade from high-grade bowel wall fibrosis in ex vivo human intestinal specimens. J Ultrasound Med. 2014; 33:2115–2123.
Article23. Fraquelli M, Branchi F, Cribiù FM, et al. The role of ultrasound elasticity imaging in predicting ileal fibrosis in Crohn’s disease patients. Inflamm Bowel Dis. 2015; 21:2605–2612.
Article24. Chen YJ, Mao R, Li XH, et al. Real-time shear wave ultrasound elastography differentiates fibrotic from inflammatory strictures in patients with Crohn’s disease. Version 2. Inflamm Bowel Dis. 2018; 24:2183–2190.
Article25. Ding SS, Fang Y, Wan J, et al. Usefulness of strain elastography, ARFI Imaging, and point shear wave elastography for the assessment of Crohn disease strictures. J Ultrasound Med. 2019; 38:2861–2870.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fibrostenotic strictures in Crohn’s disease
- Comparative Study between Axial and Coronal Planes of CT Enterography in Evaluation of Disease Activity and Complications of Crohn Disease
- Current status of endoscopic balloon dilation for Crohn's disease
- Endoscopic Balloon Dilation for Crohn’s Disease-Associated Strictures
- Imaging Techniques and Differential Diagnosis for Inflammatory Bowel Disease