Intest Res.
2020 Oct;18(4):355-378. 10.5217/ir.2019.09176.
Colitis and Crohn’s Foundation (India) consensus statements on use of 5-aminosalicylic acid in inflammatory bowel disease
- Affiliations
-
- 1Department of Gastroenterology, Dayanand Medical College and Hospital, Ludhiana, India
- 2Department of Gastroenterology, All India Institute of Medical Sciences, New Delhi, India
- 3Department of Internal Medicine, Dayanand Medical College and Hospital, Ludhiana, India
- 4Department of Gastroenterology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
- 5Department of Gastroenterology, Kasturba Medical College, Manipal, India
- 6Fortis Hospital, New Delhi, India
- 7P. D. Hinduja Hospital and Medical Research Centre, Mumbai, India
- 8Asian Institute of Gastroenterology, Hyderabad, India
- 9Department of Gastroenterology, King Edward Memorial Hospital, Mumbai, India
- 10Department of Gastroenterology, AMRI Hospital, Kolkata, India
- 11Department of Gastroenterology, Dr. Sampurnanand Medical College, Jodhpur, India
- 12Department of Gastroenterology, Moti Lal Nehru Medical College, Allahabad, India
- 13Department of Gastroenterology, Gauhati Medical College, Guwahati, India
- 14Department of Pharmacology, Dayanand Medical College and Hospital, Ludhiana, India
- 15Max Super Speciality Hospital, Saket, New Delhi, India
- 16BLK Super Speciality Hospital, New Delhi, India
- 17Citizens Centre for Digestive Disorders, Hyderabad, India
- 18Department of Gastroenterology, Sriram Chandra Bhanj Medical College and Hospital, Cuttack, India
- KMID: 2508562
- DOI: http://doi.org/10.5217/ir.2019.09176
Abstract
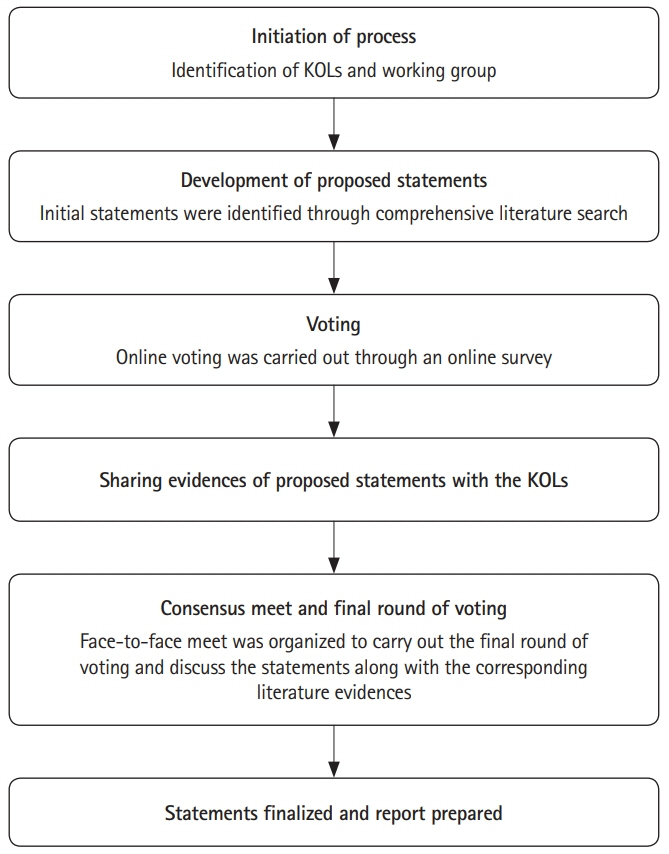
- Despite several recent advances in therapy in inflammatory bowel disease (IBD), 5-aminosalicylic acid (5-ASA) therapy has retained its place especially in ulcerative colitis. This consensus on 5-ASA is obtained through a modified Delphi process, and includes guiding statements and recommendations based on literature evidence (randomized trials, and observational studies), clinical practice, and expert opinion on use of 5-ASA in IBD by Indian gastroenterologists. The aim is to aid practitioners in selecting appropriate treatment strategies and facilitate optimal use of 5-ASA in patients with IBD.
Keyword
Figure
Cited by 2 articles
-
비전형적 궤양성 대장염 환자에서 5-아미노살리실산에 의해 유발된 심근염
Hyo Yeop Song, Geom Seog Seo
Korean J Gastroenterol. 2022;79(1):31-34. doi: 10.4166/kjg.2021.152.The impact of clinical experience on decision-making regarding the treatment and management of mild-to-moderate ulcerative colitis
Jae Hee Cheon, Kristine Paridaens, Sameer Al Awadhi, Jakob Begun, John R Fullarton, Edouard Louis, Fernando Magro, Juan Ricardo Marquez, Alexander R Moschen, Neeraj Narula, Grazyna Rydzewska, Axel U Dignass, Simon PL Travis
Intest Res. 2023;21(1):161-167. doi: 10.5217/ir.2022.00006.
Reference
-
1. Coward S, Kaplan GG. IBD in the new world, old world, and your world. In : Cohen RD, editor. Inflammatory bowel disease: diagnosis and therapeutics. 3rd ed. Cham: Springer;2017. p. 13–27.2. Singh P, Ananthakrishnan A, Ahuja V. Pivot to Asia: inflammatory bowel disease burden. Intest Res. 2017; 15:138–141.
Article3. Kedia S, Ahuja V. Epidemiology of inflammatory bowel disease in India: the great shift East. Inflamm Intest Dis. 2017; 2:102–115.
Article4. Karagozian R, Burakoff R. The role of mesalamine in the treatment of ulcerative colitis. Ther Clin Risk Manag. 2007; 3:893–903.5. Rousseaux C, Lefebvre B, Dubuquoy L, et al. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J Exp Med. 2005; 201:1205–1215.
Article6. Abdu-Allah HH, El-Shorbagi AN, Abdel-Moty SG, El-Awady R, Abdel-Alim AA. 5-Aminosalyclic acid (5-ASA): a unique anti-inflammatory salicylate. Med Chem (Los Angeles). 2016; 6:306–315.
Article7. Hanauer SB. Review article: aminosalicylates in inflammatory bowel disease. Aliment Pharmacol Ther. 2004; 20 Suppl4:60–65.8. Ye B, van Langenberg DR. Mesalazine preparations for the treatment of ulcerative colitis: are all created equal? World J Gastrointest Pharmacol Ther. 2015; 6:137–144.
Article9. Williams C, Panaccione R, Ghosh S, Rioux K. Optimizing clinical use of mesalazine (5-aminosalicylic acid) in inflammatory bowel disease. Therap Adv Gastroenterol. 2011; 4:237–248.
Article10. Ham M, Moss AC. Mesalamine in the treatment and maintenance of remission of ulcerative colitis. Expert Rev Clin Pharmacol. 2012; 5:113–123.
Article11. Sellin JH, Pasricha PJ. Pharmacotherapy of inflammatory bowel diseases. In : Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill Companies;2006. p. 1009–1011.12. D’Incà R, Paccagnella M, Cardin R, et al. 5-ASA colonic mucosal concentrations resulting from different pharmaceutical formulations in ulcerative colitis. World J Gastroenterol. 2013; 19:5665–5670.
Article13. Bodagala VR, Korlakunta NJ, Ambati SR. Design and evaluation of novel high-load mesalamine multi-particulate formulations for colon-targeted controlled drug delivery. J Compr Pharm. 2016; 3:209–219.
Article14. Böhm SK, Kruis W. Long-term efficacy and safety of oncedaily mesalazine granules for the treatment of active ulcerative colitis. Clin Exp Gastroenterol. 2014; 7:369–383.15. NHS. Oxford University Hospitals NHS Foundation Trust Web site. https://www.ouh.nhs.uk/patient-guide/leaflets/files/100722paedmesalazine.pdf. Accessed October 18, 2019.16. Sandborn WJ, Hanauer SB. Systematic review: the pharmacokinetic profiles of oral mesalazine formulations and mesalazine pro-drugs used in the management of ulcerative colitis. Aliment Pharmacol Ther. 2003; 17:29–42.
Article17. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011; 64:383–394.
Article18. Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. rating the quality of evidence: study limitations (risk of bias). J Clin Epidemiol. 2011; 64:407–415.
Article19. Canadian Task Force on the Periodic Health Examination. The periodic health examination. Can Med Assoc J. 1979; 121:1193–1254.20. Ramakrishna BS, Makharia GK, Abraham P, et al. Indian Society of Gastroenterology consensus on ulcerative colitis. Indian J Gastroenterol. 2012; 31:307–323.
Article21. Linstone H, Turoff M. The Delphi method: techniques and application. New Jersey Institute of Technology Web site. https://web.njit.edu/~turoff/pubs/delphibook/delphibook.pdf. Accessed October 18, 2019.22. Baron JH, Connell AM, Lennard-Jones JE, Jones FA. Sulphasalazine and salicylazosulphadimidine in ulcerative colitis. Lancet. 1962; 1:1094–1096.
Article23. Dick AP, Grayson MJ, Carpenter RG, Petrie A. Controlled trial of sulphasalazine in the treatment of ulcerative colitis. Gut. 1964; 5:437–442.
Article24. Nguyen NH, Fumery M, Dulai PS, et al. Comparative efficacy and tolerability of pharmacological agents for management of mild to moderate ulcerative colitis: a systematic review and network meta-analyses. Lancet Gastroenterol Hepatol. 2018; 3:742–753.
Article25. Feagan BG, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012; 10–CD000543.
Article26. Wang Y, Parker CE, Bhanji T, Feagan BG, MacDonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016; 4–CD000543.27. Probert C. Steroids and 5-aminosalicylic acids in moderate ulcerative colitis: addressing the dilemma. Therap Adv Gastroenterol. 2013; 6:33–38.
Article28. Campieri M, Adamo S, Valpiani D, et al. Oral beclometasone dipropionate in the treatment of extensive and left-sided active ulcerative colitis: a multicentre randomised study. Aliment Pharmacol Ther. 2003; 17:1471–1480.29. Suzuki Y, Iida M, Ito H, Saida I, Hibi T. Efficacy and safety of two pH-dependent-release mesalamine doses in moderately active ulcerative colitis: a multicenter, randomized, double-blind, parallel-group study. Intest Res. 2016; 14:50–59.
Article30. Mansfield JC, Giaffer MH, Cann PA, McKenna D, Thornton PC, Holdsworth CD. A double-blind comparison of balsalazide, 6.75 g, and sulfasalazine, 3 g, as sole therapy in the management of ulcerative colitis. Aliment Pharmacol Ther. 2002; 16:69–77.
Article31. Ford AC, Khan KJ, Achkar JP, Moayyedi P. Efficacy of oral vs. topical, or combined oral and topical 5-aminosalicylates, in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2012; 107:167–176.
Article32. McCormack PL, Robinson DM, Perry CM. Delayed-release multi matrix system (MMX) mesalazine: in ulcerative colitis. Drugs. 2007; 67:2635–2642.
Article33. Li W, Zhang ZM, Jiang XL. Once daily vs multiple daily mesalamine therapy for mild to moderate ulcerative colitis: a meta-analysis. Colorectal Dis. 2016; 18–O214-O223.34. Keil R, Wasserbauer M, Zádorová Z, et al. Adherence, risk factors of non-adherence and patient’s preferred treatment strategy of mesalazine in ulcerative colitis: multicentric observational study. Scand J Gastroenterol. 2018; 53:459–465.
Article35. Tong JL, Huang ML, Xu XT, Qiao YQ, Ran ZH. Once-daily versus multiple-daily mesalamine for patients with ulcerative colitis: a meta-analysis. J Dig Dis. 2012; 13:200–207.
Article36. Orchard TR, van der Geest SA, Travis SP. Randomised clinical trial: early assessment after 2 weeks of high-dose mesalazine for moderately active ulcerative colitis: new light on a familiar question. Aliment Pharmacol Ther. 2011; 33:1028–1035.
Article37. Hanauer SB, Sandborn WJ, Dallaire C, et al. Delayed-release oral mesalamine 4.8 g/day (800 mg tablets) compared to 2.4 g/day (400 mg tablets) for the treatment of mildly to moderately active ulcerative colitis: the ASCEND I trial. Can J Gastroenterol. 2007; 21:827–834.
Article38. Hanauer SB, Sandborn WJ, Kornbluth A, et al. Delayed-release oral mesalamine at 4.8 g/day (800 mg tablet) for the treatment of moderately active ulcerative colitis: the ASCEND II trial. Am J Gastroenterol. 2005; 100:2478–2485.
Article39. Sandborn WJ, Regula J, Feagan BG, et al. Delayed-release oral mesalamine 4.8 g/day (800-mg tablet) is effective for patients with moderately active ulcerative colitis. Gastroenterology. 2009; 137:1934–1943.
Article40. Peluso R, Manguso F, Vitiello M, Iervolino S, Di Minno MN. Management of arthropathy in inflammatory bowel diseases. Ther Adv Chronic Dis. 2015; 6:65–77.
Article41. Smale S, Natt RS, Orchard TR, Russell AS, Bjarnason I. Inflammatory bowel disease and spondylarthropathy. Arthritis Rheum. 2001; 44:2728–2736.
Article42. Orchard TR, Wordsworth BP, Jewell DP. Peripheral arthropathies in inflammatory bowel disease: their articular distribution and natural history. Gut. 1998; 42:387–391.
Article43. Voulgari PV. Rheumatological manifestations in inflammatory bowel disease. Ann Gastroenterol. 2011; 24:173–180.44. Lakatos PL, Lakatos L, Kiss LS, Peyrin-Biroulet L, Schoepfer A, Vavricka S. Treatment of extraintestinal manifestations in inflammatory bowel disease. Digestion. 2012; 86 Suppl 1:28–35.
Article45. Olivieri I, Cantini F, Castiglione F, et al. Italian Expert Panel on the management of patients with coexisting spondyloarthritis and inflammatory bowel disease. Autoimmun Rev. 2014; 13:822–830.
Article46. Van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017; 76:978–991.
Article47. Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011; 60:571–607.
Article48. Macken L, Blaker PA. Management of acute severe ulcerative colitis (NICE CG 166). Clin Med (Lond). 2015; 15:473–476.
Article49. Doherty G, Bennett G, Patil S, Cheifetz A, Moss AC. Interventions for prevention of post-operative recurrence of Crohn’s disease. Cochrane Database Syst Rev. 2009; (4):CD006873.
Article50. Bitton A, Buie D, Enns R, et al. Treatment of hospitalized adult patients with severe ulcerative colitis: Toronto consensus statements. Am J Gastroenterol. 2012; 107:179–194.
Article51. Feagan BG, Macdonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012; 10–CD000544.
Article52. Paoluzi OA, Iacopini F, Pica R, et al. Comparison of two different daily dosages (2.4 vs. 1.2 g) of oral mesalazine in maintenance of remission in ulcerative colitis patients: 1-year follow-up study. Aliment Pharmacol Ther. 2005; 21:1111–1119.
Article53. Wright R, Truelove SR. Serial rectal biopsy in ulcerative colitis during the course of a controlled therapeutic trial of various diets. Am J Dig Dis. 1966; 11:847–857.
Article54. Meucci G, Fasoli R, Saibeni S, et al. Prognostic significance of endoscopic remission in patients with active ulcerative colitis treated with oral and topical mesalazine: a prospective, multicenter study. Inflamm Bowel Dis. 2012; 18:1006–1010.
Article55. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011; 141:1194–1201.
Article56. Ardizzone S, Cassinotti A, Duca P, et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2011; 9:483–489.
Article57. Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007; 133:412–422.
Article58. Annese V, Daperno M, Rutter MD, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013; 7:982–1018.
Article59. Rubin DT, LoSavio A, Yadron N, Huo D, Hanauer SB. Aminosalicylate therapy in the prevention of dysplasia and colorectal cancer in ulcerative colitis. Clin Gastroenterol Hepatol. 2006; 4:1346–1350.
Article60. Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004; 126:451–459.
Article61. Zahn A, Hinz U, Karner M, Ehehalt R, Stremmel W. Healthrelated quality of life correlates with clinical and endoscopic activity indexes but not with demographic features in patients with ulcerative colitis. Inflamm Bowel Dis. 2006; 12:1058–1067.
Article62. Feagan BG, Reinisch W, Rutgeerts P, et al. The effects of infliximab therapy on health-related quality of life in ulcerative colitis patients. Am J Gastroenterol. 2007; 102:794–802.
Article63. Khan N, Abbas AM, Koleva YN, Bazzano LA. Long-term mesalamine maintenance in ulcerative colitis: which is more important? Adherence or daily dose. Inflamm Bowel Dis. 2013; 19:1123–1129.64. Fukuda T, Naganuma M, Sugimoto S, et al. The risk factor of clinical relapse in ulcerative colitis patients with low dose 5-aminosalicylic acid as maintenance therapy: a report from the IBD registry. PLoS One. 2017; 12:e0187737.
Article65. Ardizzone S, Petrillo M, Imbesi V, Cerutti R, Bollani S, Bianchi Porro G. Is maintenance therapy always necessary for patients with ulcerative colitis in remission? Aliment Pharmacol Ther. 1999; 13:373–379.
Article66. Takahashi F, Tominaga K, Kanamori A, et al. Timing for dose-down of 5-ASA depends on mucosal status with ulcerative colitis. Scand J Gastroenterol. 2016; 51:827–834.
Article67. Rubin DT, Bradette M, Gabalec L, et al. Ulcerative colitis remission status after induction with mesalazine predicts maintenance outcomes: the MOMENTUM trial. J Crohns Colitis. 2016; 10:925–933.
Article68. Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005; 100:1345–1353.
Article69. Fockens P, Mulder CJ, Tytgat GN, et al. Comparison of the efficacy and safety of 1.5 compared with 3.0 g oral slow-release mesalazine (Pentasa) in the maintenance treatment of ulcerative colitis. Dutch Pentasa Study Group. Eur J Gastroenterol Hepatol. 1995; 7:1025–1030.
Article70. Mañosa M, Cabré E, Garcia-Planella E, et al. Decision tree for early introduction of rescue therapy in active ulcerative colitis treated with steroids. Inflamm Bowel Dis. 2011; 17:2497–2502.
Article71. Faubion WA Jr, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001; 121:255–260.
Article72. Ho GT, Chiam P, Drummond H, Loane J, Arnott ID, Satsangi J. The efficacy of corticosteroid therapy in inflammatory bowel disease: analysis of a 5-year UK inception cohort. Aliment Pharmacol Ther. 2006; 24:319–330.
Article73. Bello C, Belaiche J, Louis E, Reenaers C. Evolution and predictive factors of relapse in ulcerative colitis patients treated with mesalazine after a first course of corticosteroids. J Crohns Colitis. 2011; 5:196–202.
Article74. Khan N, Abbas A, Williamson A, Balart L. Prevalence of corticosteroids use and disease course after initial steroid exposure in ulcerative colitis. Dig Dis Sci. 2013; 58:2963–2969.
Article75. Garcia-Planella E, Mañosa M, Van Domselaar M, et al. Long-term outcome of ulcerative colitis in patients who achieve clinical remission with a first course of corticosteroids. Dig Liver Dis. 2012; 44:206–210.
Article76. Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010; 105:501–523.
Article77. Sninsky CA. New research in ulcerative colitis: optimizing 5-ASA administration for efficacy and adherence. Gastroenterol Hepatol (N Y). 2010; 6(1 Suppl 1):4–16.78. Marteau P, Crand J, Foucault M, Rambaud JC. Use of mesalazine slow release suppositories 1 g three times per week to maintain remission of ulcerative proctitis: a randomised double blind placebo controlled multicentre study. Gut. 1998; 42:195–199.
Article79. Hanauer S, Good LI, Goodman MW, et al. Long-term use of mesalamine (Rowasa) suppositories in remission maintenance of ulcerative proctitis. Am J Gastroenterol. 2000; 95:1749–1754.
Article80. Lie MR, Kanis SL, Hansen BE, van der Woude CJ. Drug therapies for ulcerative proctitis: systematic review and meta-analysis. Inflamm Bowel Dis. 2014; 20:2157–2178.81. Ford AC, Khan KJ, Sandborn WJ, Hanauer SB, Moayyedi P. Efficacy of topical 5-aminosalicylates in preventing relapse of quiescent ulcerative colitis: a meta-analysis. Clin Gastroenterol Hepatol. 2012; 10:513–519.
Article82. Marshall JK, Thabane M, Steinhart AH, Newman JR, Anand A, Irvine EJ. Rectal 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012; 11–CD004118.
Article83. d’Albasio G, Pacini F, Camarri E, et al. Combined therapy with 5-aminosalicylic acid tablets and enemas for maintaining remission in ulcerative colitis: a randomized double-blind study. Am J Gastroenterol. 1997; 92:1143–1147.84. Yokoyama H, Takagi S, Kuriyama S, et al. Effect of weekend 5-aminosalicylic acid (mesalazine) enema as maintenance therapy for ulcerative colitis: results from a randomized controlled study. Inflamm Bowel Dis. 2007; 13:1115–1120.
Article85. Moody GA, Eaden JA, Helyes Z, Mayberry JF. Oral or rectal administration of drugs in IBD? Aliment Pharmacol Ther. 1997; 11:999–1000.
Article86. Boyle M, Ting A, Cury DB, Nanda K, Cheifetz AS, Moss A. Adherence to rectal mesalamine in patients with ulcerative colitis. Inflamm Bowel Dis. 2015; 21:2873–2878.
Article87. Kamm MA, Lichtenstein GR, Sandborn WJ, et al. Randomised trial of once- or twice-daily MMX mesalazine for maintenance of remission in ulcerative colitis. Gut. 2008; 57:893–902.
Article88. Bokemeyer B, Hommes D, Gill I, Broberg P, Dignass A. Mesalazine in left-sided ulcerative colitis: efficacy analyses from the PODIUM trial on maintenance of remission and mucosal healing. J Crohns Colitis. 2012; 6:476–482.
Article89. Marshall JK, Irvine EJ. Rectal aminosalicylate therapy for distal ulcerative colitis: a meta-analysis. Aliment Pharmacol Ther. 1995; 9:293–300.
Article90. Seibold F, Fournier N, Beglinger C, et al. Topical therapy is underused in patients with ulcerative colitis. J Crohns Colitis. 2014; 8:56–63.
Article91. Römkens TE, Kampschreur MT, Drenth JP, van Oijen MG, de Jong DJ. High mucosal healing rates in 5-ASA-treated ulcerative colitis patients: results of a meta-analysis of clinical trials. Inflamm Bowel Dis. 2012; 18:2190–2198.
Article92. Farup PG, Hovde O, Halvorsen FA, Raknerud N, Brodin U. Mesalazine suppositories versus hydrocortisone foam in patients with distal ulcerative colitis: a comparison of the efficacy and practicality of two topical treatment regimens. Scand J Gastroenterol. 1995; 30:164–170.
Article93. Campieri M, Gionchetti P, Belluzzi A, et al. 5-Aminosalicylic acid as enemas or suppositories in distal ulcerative colitis? J Clin Gastroenterol. 1988; 10:406–409.
Article94. Regueiro M, Loftus EV Jr, Steinhart AH, Cohen RD. Medical management of left-sided ulcerative colitis and ulcerative proctitis: critical evaluation of therapeutic trials. Inflamm Bowel Dis. 2006; 12:979–994.
Article95. Lamet M. A multicenter, randomized study to evaluate the efficacy and safety of mesalamine suppositories 1 g at bedtime and 500 mg twice daily in patients with active mild-to-moderate ulcerative proctitis. Dig Dis Sci. 2011; 56:513–522.
Article96. Andus T, Kocjan A, Müser M, et al. Clinical trial: a novel high-dose 1 g mesalamine suppository (Salofalk) once daily is as efficacious as a 500-mg suppository thrice daily in active ulcerative proctitis. Inflamm Bowel Dis. 2010; 16:1947–1956.
Article97. Marshall JK, Thabane M, Steinhart AH, Newman JR, Anand A, Irvine EJ. Rectal 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2010; (1):CD004115.
Article98. Gionchetti P, Rizzello F, Venturi A, et al. Comparison of oral with rectal mesalazine in the treatment of ulcerative proctitis. Dis Colon Rectum. 1998; 41:93–97.
Article99. Caselli MG, Pinedo MG, Zúñiga DA, Alvarez LM. Active and refractory ulcerative proctitis: an update. Rev Med Chil. 2010; 138:109–116.100. Safdi M, DeMicco M, Sninsky C, et al. A double-blind comparison of oral versus rectal mesalamine versus combination therapy in the treatment of distal ulcerative colitis. Am J Gastroenterol. 1997; 92:1867–1871.101. Probert CS, Dignass AU, Lindgren S, Oudkerk Pool M, Marteau P. Combined oral and rectal mesalazine for the treatment of mild-to-moderately active ulcerative colitis: rapid symptom resolution and improvements in quality of life. J Crohns Colitis. 2014; 8:200–207.
Article102. Marshall JK, Irvine EJ. Rectal corticosteroids versus alternative treatments in ulcerative colitis: a meta-analysis. Gut. 1997; 40:775–781.
Article103. Mulder CJ, Fockens P, Meijer JW, van der Heide H, Wiltink EH, Tytgat GN. Beclomethasone dipropionate (3 mg) versus 5-aminosalicylic acid (2 g) versus the combination of both (3 mg/2 g) as retention enemas in active ulcerative proctitis. Eur J Gastroenterol Hepatol. 1996; 8:549–553.104. Nagahori M, Kochi S, Hanai H, et al. Real life results in using 5-ASA for maintaining mild to moderate UC patients in Japan, a multi-center study, OPTIMUM Study. BMC Gastroenterol. 2017; 17:47.
Article105. Lee HJ, Jung ES, Lee JH, et al. Long-term clinical outcomes and factors predictive of relapse after 5-aminosalicylate or sulfasalazine therapy in patients with mild-to-moderate ulcerative colitis. Hepatogastroenterology. 2012; 59:1415–1420.106. Ransford RA, Langman MJ. Sulphasalazine and mesalazine: serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut. 2002; 51:536–539.
Article107. Narang V, Kaur R, Garg B, et al. Association of endoscopic and histological remission with clinical course in patients of ulcerative colitis. Intest Res. 2018; 16:55–61.
Article108. Van Hees PA, van Lier HJ, van Elteren PH, et al. Effect of sulphasalazine in patients with active Crohn’s disease: a controlled double-blind study. Gut. 1981; 22:404–409.
Article109. Singleton JW. Results of treatment with sulfasalazine in the American multicenter study on the treatment of Crohn disease (National Cooperative Crohn’s Disease Study). Z Gastroenterol Verh. 1981; 19:38–40.110. Malchow H, Ewe K, Brandes JW, et al. European Cooperative Crohn’s Disease Study (ECCDS): results of drug treatment. Gastroenterology. 1984; 86:249–266.
Article111. Lim WC, Wang Y, MacDonald JK, Hanauer S. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst Rev. 2016; 7–CD008870.
Article112. Lindsay JO, Irving PM, Mantzaris GJ, Panés J; ECCO Education Committee and ECCO Governing Board. ECCO IBD Curriculum. J Crohns Colitis. 2017; 11:1039–1043.
Article113. Sobrado CW, Leal RF, Sobrado LF. Therapies for Crohn’s disease: a clinical update. Arq Gastroenterol. 2016; 53:206–211.
Article114. Gordon M. 5-Aminosalicylates to maintain remission in Crohn’s disease: interpreting conflicting systematic review evidence. World J Gastrointest Pharmacol Ther. 2017; 8:99–102.
Article115. Bradley GM, Oliva-Hemker M. Pediatric ulcerative colitis: current treatment approaches including role of infliximab. Biologics. 2012; 6:125–134.116. Sokollik C, Fournier N, Rizzuti D, et al. The use of 5-aminosalicylic acid in children and adolescents with inflammatory bowel disease. J Clin Gastroenterol. 2018; 52:e87–e91.
Article117. Levine A, Yerushalmi B, Kori M, et al. Mesalamine enemas for induction of remission in oral mesalamine-refractory pediatric ulcerative colitis: a prospective cohort study. J Crohns Colitis. 2017; 11:970–974.
Article118. Winter HS, Krzeski P, Heyman MB, et al. High- and low-dose oral delayed-release mesalamine in children with mild-to-moderately active ulcerative colitis. J Pediatr Gastroenterol Nutr. 2014; 59:767–772.
Article119. Turner D, Yerushalmi B, Kori M, et al. Once- versus twice-daily mesalazine to induce remission in paediatric ulcerative colitis: a randomised controlled trial. J Crohns Colitis. 2017; 11:527–533.
Article120. Ford AC, Achkar JP, Khan KJ, et al. Efficacy of 5-aminosalicylates in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2011; 106:601–616.
Article121. Quiros JA, Heyman MB, Pohl JF, et al. Safety, efficacy, and pharmacokinetics of balsalazide in pediatric patients with mild-to-moderate active ulcerative colitis: results of a randomized, double-blind study. J Pediatr Gastroenterol Nutr. 2009; 49:571–579.
Article122. Ferry GD, Kirschner BS, Grand RJ, et al. Olsalazine versus sulfasalazine in mild to moderate childhood ulcerative colitis: results of the Pediatric Gastroenterology Collaborative Research Group Clinical Trial. J Pediatr Gastroenterol Nutr. 1993; 17:32–38.
Article123. Heyman MB, Kierkus J, Spénard J, Shbaklo H, Giguere M. Efficacy and safety of mesalamine suppositories for treatment of ulcerative proctitis in children and adolescents. Inflamm Bowel Dis. 2010; 16:1931–1939.
Article124. Nikfar S, Rahimi R, Rezaie A, Abdollahi M. A meta-analysis of the efficacy of sulfasalazine in comparison with 5-aminosalicylates in the induction of improvement and maintenance of remission in patients with ulcerative colitis. Dig Dis Sci. 2009; 54:1157–1170.
Article125. Cuffari C, Pierce D, Korczowski B, et al. Randomized clinical trial: pharmacokinetics and safety of multimatrix mesalamine for treatment of pediatric ulcerative colitis. Drug Des Devel Ther. 2016; 10:593–607.126. Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Pregnancy outcome in women with inflammatory bowel disease following exposure to 5-aminosalicylic acid drugs: a meta-analysis. Reprod Toxicol. 2008; 25:271–275.
Article127. Cury DB, Moss AC. Treatment of Crohn’s disease in pregnant women: drug and multidisciplinary approaches. World J Gastroenterol. 2014; 20:8790–8795.128. Hendy P, Chadwick G, Hart A. IBD: reproductive health, pregnancy and lactation. Frontline Gastroenterol. 2015; 6:38–43.
Article129. Habal FM, Kapila V. Inflammatory bowel disease and pregnancy: evidence, uncertainty and patient decision-making. Can J Gastroenterol. 2009; 23:49–53.
Article130. Gallinger ZR, Nguyen GC. Presence of phthalates in gastrointestinal medications: is there a hidden danger? World J Gastroenterol. 2013; 19:7042–7047.
Article131. van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015; 9:107–124.
Article132. Silverman DA, Ford J, Shaw I, Probert CS. Is mesalazine really safe for use in breastfeeding mothers? Gut. 2005; 54:170–171.
Article133. Nielsen OH, Maxwell C, Hendel J. IBD medications during pregnancy and lactation. Nat Rev Gastroenterol Hepatol. 2014; 11:116–127.
Article134. Loftus EV Jr, Kane SV, Bjorkman D. Systematic review: short-term adverse effects of 5-aminosalicylic acid agents in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2004; 19:179–189.
Article135. Scheurlen C, Allgayer H, Kruis W, Erdmann E, Sauerbruch T. Effect of olsalazine and mesalazine on human ileal and colonic (Na+ + K+)-ATPase: a possible diarrhogenic factor? Clin Investig. 1993; 71:286–289.136. Muller AF, Stevens PE, McIntyre AS, Ellison H, Logan RF. Experience of 5-aminosalicylate nephrotoxicity in the United Kingdom. Aliment Pharmacol Ther. 2005; 21:1217–1224.
Article137. Gisbert JP, González-Lama Y, Maté J. 5-Aminosalicylates and renal function in inflammatory bowel disease: a systematic review. Inflamm Bowel Dis. 2007; 13:629–638.138. Oikonomou KA, Kapsoritakis AN, Stefanidis I, Potamianos SP. Drug-induced nephrotoxicity in inflammatory bowel disease. Nephron Clin Pract. 2011; 119:c89–c96.
Article139. Popoola J, Muller AF, Pollock L, O’Donnell P, Carmichael P, Stevens P. Late onset interstitial nephritis associated with mesalazine treatment. BMJ. 1998; 317:795–797.
Article140. Siddique N, Farmer C, Muller AF. Do gastroenterologists monitor their patients taking 5-amino-salicylates following initiation of treatment. Frontline Gastroenterol. 2015; 6:27–31.
Article141. World MJ, Stevens PE, Ashton MA, Rainford DJ. Mesalazine-associated interstitial nephritis. Nephrol Dial Transplant. 1996; 11:614–621.
Article142. The Medicines Healthcare Regulatory Authority (MHRA). Pentasa slow release tablets 1g (mesalazine). https://www.medicines.org.uk/emc/product/4778/smpc. Updated October 2019. Accessed April 28, 2020.143. Patel H, Barr A, Jeejeebhoy KN. Renal effects of long-term treatment with 5-aminosalicylic acid. Can J Gastroenterol. 2009; 23:170–176.
Article144. Tekin F, Ozutemiz O, Ilter T. 5-aminosalicylates in inflammatory bowel disease and chronic renal failure. Aliment Pharmacol Ther. 2005; 22:579.
Article145. Zallot C, Billioud V, Frimat L, Faure P, Peyrin-Biroulet L; CREGG (Club de Reflexion des cabinets et Groupes d’Hépato-Gastroentérologie). 5-Aminosalicylates and renal function monitoring in inflammatory bowel disease: a nationwide survey. J Crohns Colitis. 2013; 7:551–555.
Article146. Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005; 16:180–188.
Article147. Frimat L, Siewe G, Loos-Ayav C, Briançon S, Kessler M, Aubrège A. Chronic kidney disease: do generalists and nephrologists differ in their care? Nephrol Ther. 2006; 2:127–135.148. Chatzinoff M, Guarino JM, Corson SL, Batzer FR, Friedman LS. Sulfasalazine-induced abnormal sperm penetration assay reversed on changing to 5-aminosalicylic acid enemas. Dig Dis Sci. 1988; 33:108–110.
Article149. Birnie GG, McLeod TI, Watkinson G. Incidence of sulphasalazine-induced male infertility. Gut. 1981; 22:452–455.
Article150. Mahadevan U. Fertility and pregnancy in the patient with inflammatory bowel disease. Gut. 2006; 55:1198–1206.
Article151. Sands K, Jansen R, Zaslau S, Greenwald D. Review article: the safety of therapeutic drugs in male inflammatory bowel disease patients wishing to conceive. Aliment Pharmacol Ther. 2015; 41:821–834.
Article152. Vermeire S, Carbonnel F, Coulie PG, et al. Management of inflammatory bowel disease in pregnancy. J Crohns Colitis. 2012; 6:811–823.
Article153. Halsted CH, Gandhi G, Tamura T. Sulfasalazine inhibits the absorption of folates in ulcerative colitis. N Engl J Med. 1981; 305:1513–1517.
Article154. Beu J. Chemoprevention of colorectal cancer with 5-aminosalicylic acids in adult patients with inflammatory bowel disease. Semantic Scholar Web site. https://pdfs.semanticscholar.org/8176/442c71633e61fb83e5bcd4333c369ac1bdb6.pdf. Updated August 14, 2010. Accessed October 18, 2019.155. Qiu X, Ma J, Wang K, Zhang H. Chemopreventive effects of 5-aminosalicylic acid on inflammatory bowel disease-associated colorectal cancer and dysplasia: a systematic review with meta-analysis. Oncotarget. 2017; 8:1031–1045.
Article156. Zhao LN, Li JY, Yu T, Chen GC, Yuan YH, Chen QK. 5-Aminosalicylates reduce the risk of colorectal neoplasia in patients with ulcerative colitis: an updated meta-analysis. PLoS One. 2014; 9:e94208.
Article157. Bonovas S, Fiorino G, Lytras T, Nikolopoulos G, Peyrin-Biroulet L, Danese S. Systematic review with meta-analysis: use of 5-aminosalicylates and risk of colorectal neoplasia in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017; 45:1179–1192.
Article158. Bernstein CN, Nugent Z, Blanchard JF. 5-aminosalicylate is not chemoprophylactic for colorectal cancer in IBD: a population based study. Am J Gastroenterol. 2011; 106:731–736.
Article159. Bhatt J, Patil S, Joshi A, Abraham P, Desai D. Self-reported treatment adherence in inflammatory bowel disease in Indian patients. Indian J Gastroenterol. 2009; 28:143–146.
Article160. Tomar SK, Kedia S, Singh N, et al. Higher education, professional occupation, and upper socioeconomic status are associated with lower adherence to medications in patients with inflammatory bowel disease. JGH Open. 2019; 3:302–309.
Article161. Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med. 2003; 114:39–43.
Article162. Robinson A, Hankins M, Wiseman G, Jones M. Maintaining stable symptom control in inflammatory bowel disease: a retrospective analysis of adherence, medication switches and the risk of relapse. Aliment Pharmacol Ther. 2013; 38:531–538.
Article