Anesth Pain Med.
2020 Oct;15(4):478-485. 10.17085/apm.20032.
Antinociceptive effects of intrathecal cimifugin treatment: a preliminary rat study based on formalin test
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, School of Medicine, Chosun University, Gwangju, Korea
- 2Department of Anesthesiology and Pain Medicine, Chosun University Hospital, Gwangju, Korea
- 3Department of Medicine, Graduate School, Chosun University, Gwangju, Korea
- KMID: 2508411
- DOI: http://doi.org/10.17085/apm.20032
Abstract
- Background
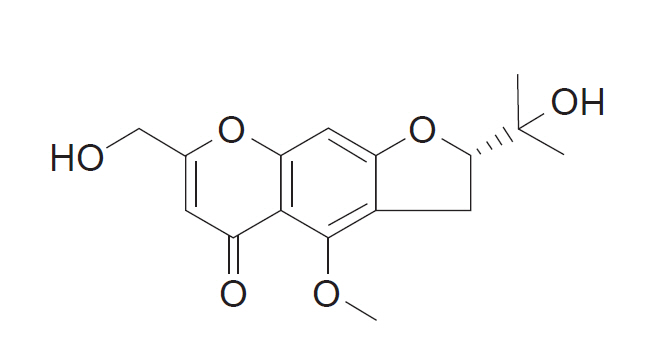
Cimifugin is one of the components of the root of Saposhnikovia divaricata. The extract derived from S. divaricata is traditionally used as an analgesic. This study was conducted to evaluate the analgesic effect of intrathecal cimifugin in the formalin test.
Methods
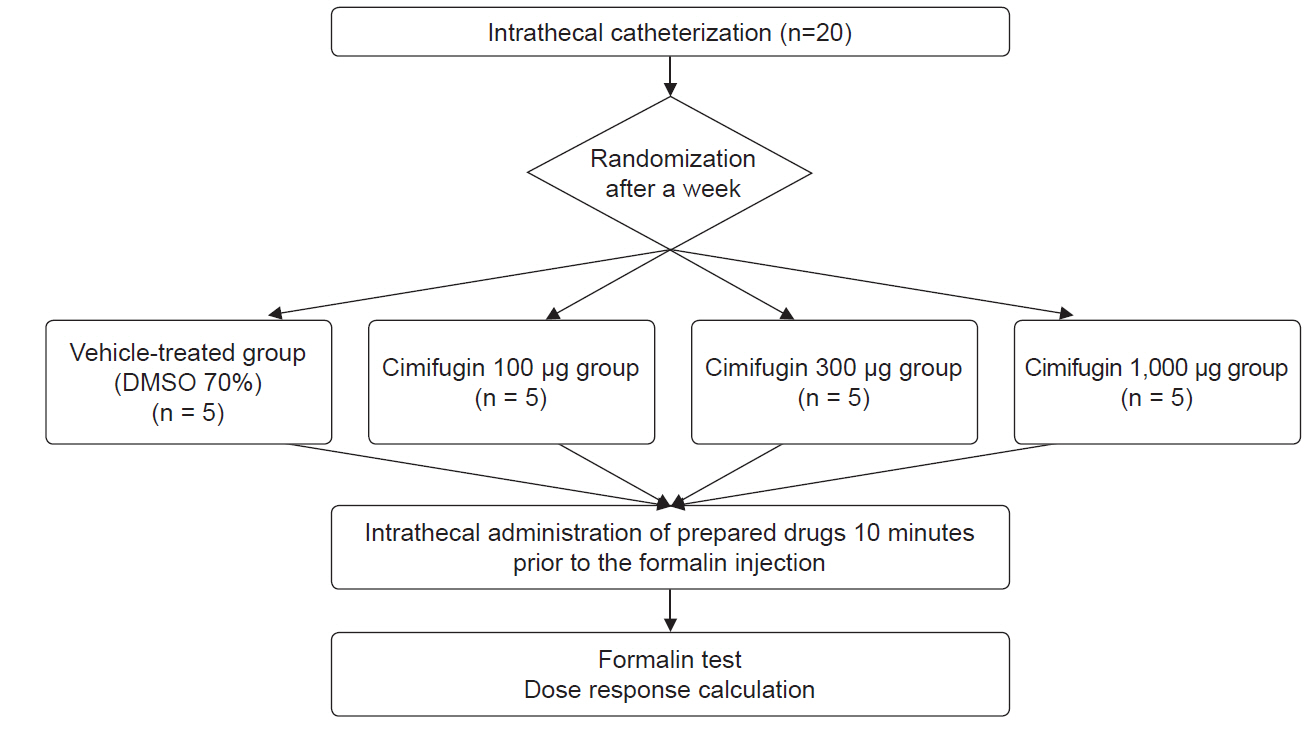
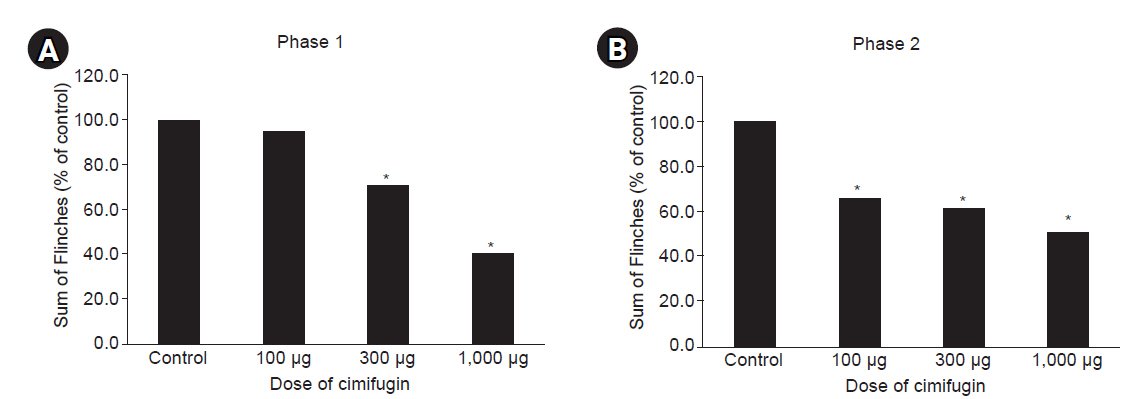
Male Sprague–Dawley rats (n = 20) were randomized into four groups for intrathecal administration of 70% dimethylsulfoxide and various doses of cimifugin (100 μg, 300 μg, and 1,000 μg). The typical flinch response after the injection of 5% formalin into the hind paw was assessed in two distinct phases: phase 1 until 10 min, and phase 2 from 10 min to 60 min. ED50 values were calculated via linear regression.
Results
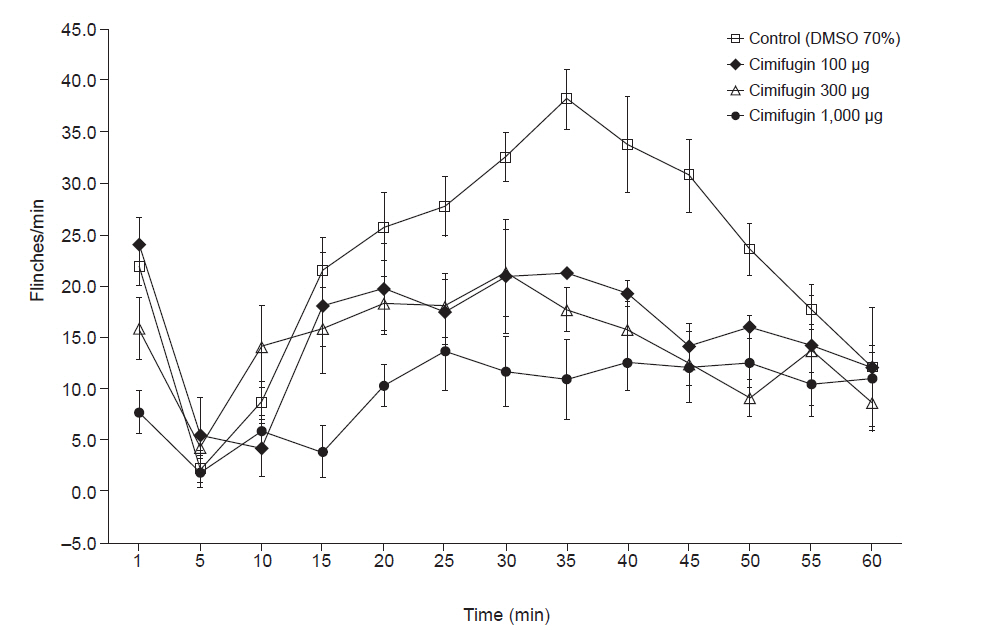
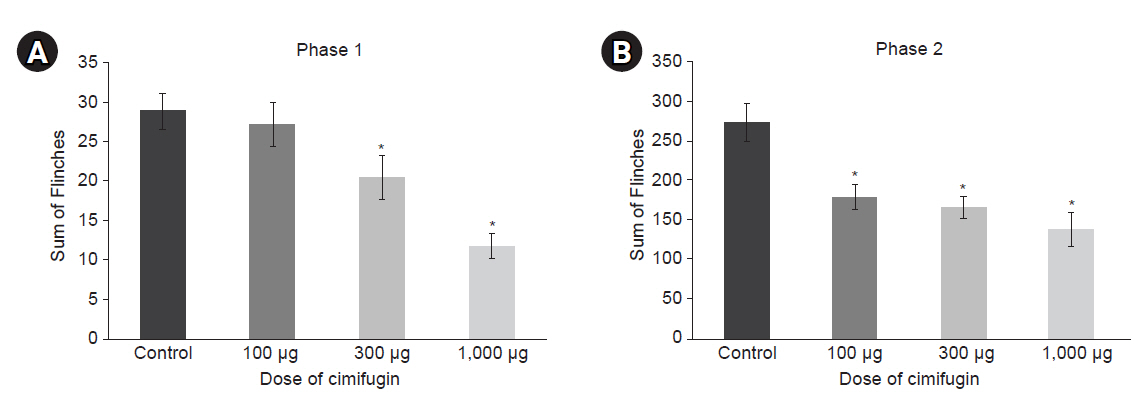
Intrathecal cimifugin significantly reduced the flinch response in both phases of the formalin test. Significant antinociceptive effects of cimifugin were found with the dose of 300 μg in phase 1 and the dose of 100 μg in phase 2. The ED50 value (95% confidence intervals) of intrathecal cimifugin was 696.1 (360.8–1,342.8) μg during phase 1 and 1,242.8 (42.0–48,292.5) μg during phase 2.
Conclusions
Intrathecal cimifugin has an antinociceptive effect against formalin-induced pain. Cimifugin has an anti-inflammatory effect at low concentrations, and non-inflammatory analgesic effect at higher concentrations.
Figure
Reference
-
1. Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001; 24:450–5.2. Gautam R, Jachak SM. Recent developments in anti-inflammatory natural products. Med Res Rev. 2009; 29:767–820.3. Vijaya Bhargavi M, Shashikala P, Sumakanth M. Coumarins and chromones: a remarkable scaffolds for anti-inflammatory activity. J Pharm Sci Res. 2017; 9:1483–9.4. Li Y, Zhao L, Zhang H, Jia J, Lv L, Zhou G, et al. Comparative pharmacokinetics of prim-O-glucosylcimifugin and cimifugin by liquid chromatography-mass spectrometry after oral administration of Radix Saposhnikoviae extract, cimifugin monomer solution and prim-O-glucosylcimifugin monomer solution to rats. Biomed Chromatogr. 2012; 26:1234–40.5. Hisamoto M, Kikuzaki H, Ohigashi H, Nakatani N. Antioxidant compounds from the leaves of Peucedanum japonicum thunb. J Agric Food Chem. 2003; 51:5255–61.6. Zimecki M, Artym J, Cisowski W, Mazol I, Włodarczyk M, Gleńsk M. Immunomodulatory and anti-inflammatory activity of selected osthole derivatives. Z Naturforsch C J Biosci. 2009; 64:361–8.7. Zheng M, Jin W, Son KH, Chang HW, Kim HP, Bae K, et al. The constituents isolated from peucedanum japonicum Thunb. and their cyclooxygenase (COX) inhibitory activity. Korean J Medicinal Crop Sci. 2005; 13:75–9.8. Okuyama E, Hasegawa T, Matsushita T, Fujimoto H, Ishibashi M, Yamazaki M. Analgesic components of saposhnikovia root (Saposhnikovia divaricata). Chem Pharm Bull (Tokyo). 2001; 49:154–60.9. Kim SH, Jong HS, Yoon MH, Oh SH, Jung KT. Antinociceptive effect of intrathecal sec-O-glucosylhamaudol on the formalin-induced pain in rats. Korean J Pain. 2017; 30:98–103.10. Wang X, Jiang X, Yu X, Liu H, Tao Y, Jiang G, et al. Cimifugin suppresses allergic inflammation by reducing epithelial derived initiative key factors via regulating tight junctions. J Cell Mol Med. 2017; 21:2926–36.11. Han B, Dai Y, Wu H, Zhang Y, Wan L, Zhao J, et al. Cimifugin inhibits inflammatory responses of RAW264.7 cells induced by lipopolysaccharide. Med Sci Monit. 2019; 25:409–17.12. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983; 16:109–10.13. Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976; 17:1031–6.14. Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996; 64:345–55.15. Tallarida RJ, Murray RB. Manual of pharmacologic calculations with computer programs. 2nd ed. New York: Springer;1987. p. 1–95.16. Li Y, Wang H, Chen J, Zhao L, Zhang H, Chai Y, et al. RRLC-TOF/MS in identification of constituents and metabolites of Radix Saposhnikoviae in rat plasma and urine. Acad J Second Mil Med Univ. 2010; 30:760–3.17. Seol TK, Lee W, Park S, Kim KN, Kim TY, Oh YN, et al. Effect of palmitoylethanolamide on inflammatory and neuropathic pain in rats. Korean J Anesthesiol. 2017; 70:561–6.18. Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987; 30:103–14.19. Park SH, Sim YB, Lee JK, Kim SM, Kang YJ, Jung JS, et al. The analgesic effects and mechanisms of orally administered eugenol. Arch Pharm Res. 2011; 34:501–7.20. Koh GH, Song H, Kim SH, Yoon MH, Lim KJ, Oh SH, et al. Effect of sec-O-glucosylhamaudol on mechanical allodynia in a rat model of postoperative pain. Korean J Pain. 2019; 32:87–96.21. Hacimuftuoglu A, Handy CR, Goettl VM, Lin CG, Dane S, Stephens RL Jr. Antioxidants attenuate multiple phases of formalin-induced nociceptive response in mice. Behav Brain Res. 2006; 173:211–6.22. Hong BH, Ko YK, Lee YJ, Han K, Kim Y, Lee W. Antinociceptive effects of vitamin E in formalin-induced nociceptive response in rats. Anesth Pain Med. 2011; 6:59–62.23. Colucci M, Maione F, Bonito MC, Piscopo A, Di Giannuario A, Pieretti S. New insights of dimethyl sulphoxide effects (DMSO) on experimental in vivo models of nociception and inflammation. Pharmacol Res. 2008; 57:419–25.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antinociceptive Effects of Intraperitoneal and Intrathecal Vitamin E in the Rat Formalin Test
- Effects of Intrathecal Flecainide for Pain Behavior in Formalin Test and Hemodynamics in Rat
- Antinociceptive drug interaction between intrathecal vitamin E and gabapentin in the rat formalin test
- Effect of Serotonergic Receptors on the Antinociception of Intrathecal Gabapentin in the Formalin Test of Rats
- Comparison of Antinociceptive Effect of Pre- versus Post-treatment with Intrathecal Magnesium on the Formalin Test in Rats