Cancer Res Treat.
2020 Oct;52(4):1153-1161. 10.4143/crt.2020.173.
A Multi-cohort Study of the Prognostic Significance of Microsatellite Instability or Mismatch Repair Status after Recurrence of Resectable Gastric Cancer
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Surgery, Yonsei University Health System, Seoul, Korea
- 3Yonsei Biomedical Research Institute, Yonsei University Health System, Seoul, Korea
- 4Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 6YUHS-KRIBB Medical Convergence Research Institute, Seoul, Korea
- 7Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2507941
- DOI: http://doi.org/10.4143/crt.2020.173
Abstract
- Purpose
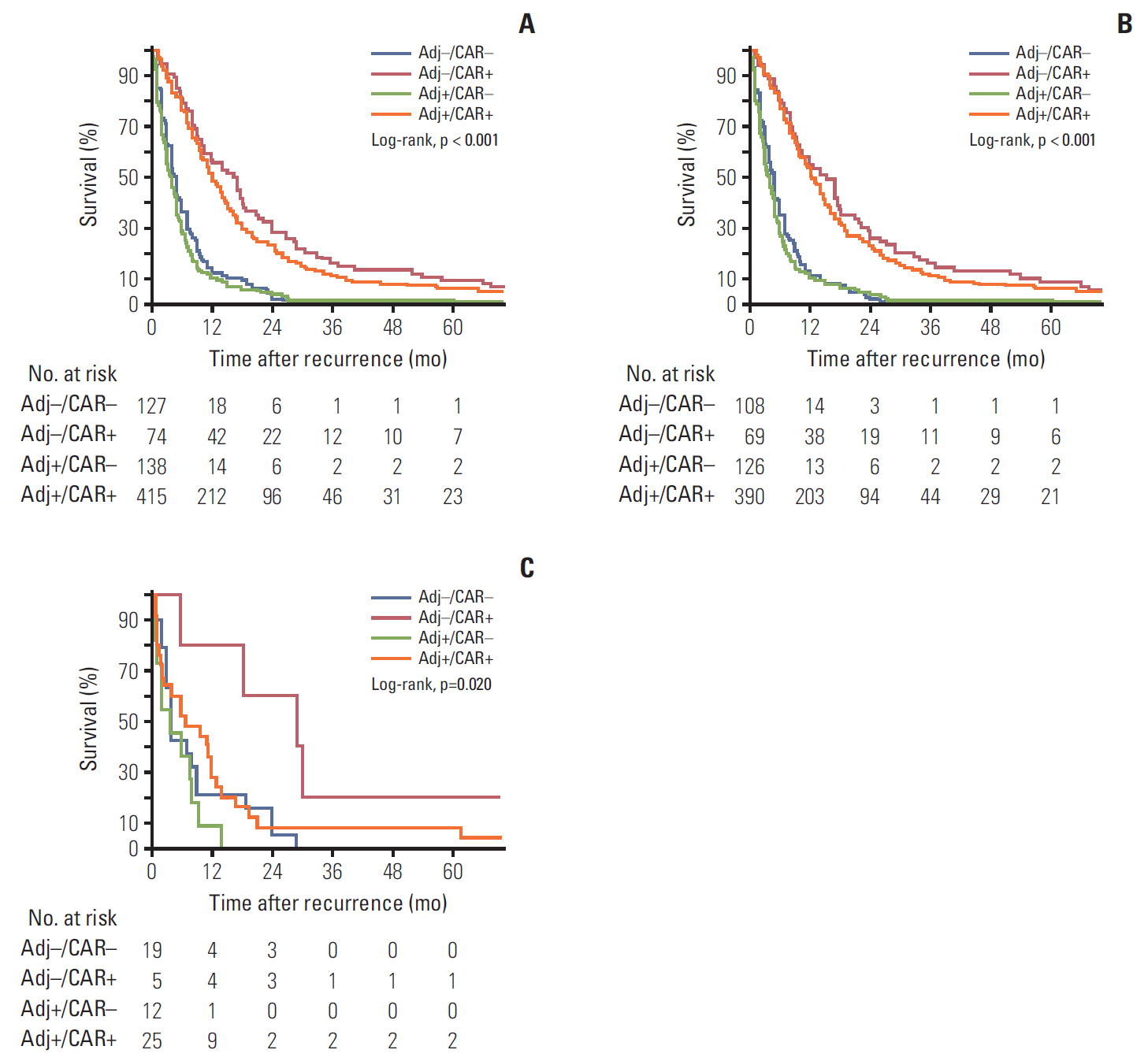
High microsatellite instability (MSI) is related to good prognosis in gastric cancer. We aimed to identify the prognostic factors of patients with recurrent gastric cancer and investigate the role of MSI as a prognostic and predictive biomarker of survival after tumor recurrence.
Materials and Methods
This retrospective cohort study enrolled patients treated for stage II/III gastric cancer who developed tumor recurrence and in whom the MSI status or mismatch repair (MMR) status of the tumor was known. MSI status and the expression of MMR proteins were evaluated using polymerase chain reaction and immunohistochemical analysis, respectively.
Results
Of the 790 patients included, 64 (8.1%) had high MSI status or MMR deficiency. The tumor-node-metastasis stage, type of recurrence, Lauren classification, chemotherapy after recurrence, and interval to recurrence were independently associated with survival after tumor recurrence. The MSI/MMR status and receiving adjuvant chemotherapy were not associated with survival after recurrence. In a subgroup analysis of patients with high MSI or MMR-deficient gastric cancer, those who did not receive adjuvant chemotherapy had better treatment response to chemotherapy after recurrence than those who received adjuvant chemotherapy.
Conclusion
Patients with high MSI/MMR-deficient gastric cancer should be spared from adjuvant chemotherapy after surgery, but aggressive chemotherapy after recurrence should be considered. Higher tumor-node-metastasis stage, Lauren classification, interval to recurrence, and type of recurrence are associated with survival after tumor recurrence and should thus be considered when establishing a treatment plan and designing clinical trials targeting recurrent gastric cancer.
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019; 51:417–30.
Article3. Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15:1389–96.
Article4. Pietrantonio F, Miceli R, Raimondi A, Kim YW, Kang WK, Langley RE, et al. Individual patient data meta-analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol. 2019; 37:3392–400.
Article5. Ito S, Ohashi Y, Sasako M. Survival after recurrence in patients with gastric cancer who receive S-1 adjuvant chemotherapy: exploratory analysis of the ACTS-GC trial. BMC Cancer. 2018; 18:449.
Article6. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014; 513:202–9.7. Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015; 21:449–56.
Article8. Cheong JH, Yang HK, Kim H, Kim WH, Kim YW, Kook MC, et al. Predictive test for chemotherapy response in resectable gastric cancer: a multi-cohort, retrospective analysis. Lancet Oncol. 2018; 19:629–38.
Article9. Yong WP, Rha SY, Tan IB, Choo SP, Syn NL, Koh V, et al. Real-time tumor gene expression profiling to direct gastric cancer chemotherapy: proof-of-concept “3G” trial. Clin Cancer Res. 2018; 24:5272–81.
Article10. Roh CK, Choi YY, Choi S, Seo WJ, Cho M, Jang E, et al. Single patient classifier assay, microsatellite instability, and Epstein-Barr virus status predict clinical outcomes in stage II/III gastric cancer: results from CLASSIC trial. Yonsei Med J. 2019; 60:132–9.
Article11. Choi YY, Kim H, Shin SJ, Kim HY, Lee J, Yang HK, et al. Microsatellite instability and programmed cell death-ligand 1 expression in stage II/III gastric cancer: post hoc analysis of the CLASSIC randomized controlled study. Ann Surg. 2019; 270:309–16.12. Smyth EC, Wotherspoon A, Peckitt C, Gonzalez D, HulkkiWilson S, Eltahir Z, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial. JAMA Oncol. 2017; 3:1197–203.13. Choi YY, Cheong JH. Comment on “To treat, or not to treat, that is the question: biomarker-guided adjuvant chemotherapy for stage II and III gastric cancer”. Ann Surg. 2019; 270:e40–1.
Article14. O’Connell MJ, Campbell ME, Goldberg RM, Grothey A, Seitz JF, Benedetti JK, et al. Survival following recurrence in stage II and III colon cancer: findings from the ACCENT data set. J Clin Oncol. 2008; 26:2336–41.15. Kim SY, Choi YY, An JY, Shin HB, Jo A, Choi H, et al. The benefit of microsatellite instability is attenuated by chemotherapy in stage II and stage III gastric cancer: results from a large cohort with subgroup analyses. Int J Cancer. 2015; 137:819–25.
Article16. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. New York: Springer;2017.17. Guideline Committee of the Korean Gastric Cancer Association (KGCA); Development Working Group and Review Panel. Korean practice guideline for gastric cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer. 2019; 19:1–48.18. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017; 20:1–19.19. Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998; 58:5248–57.20. Cho J, Kang SY, Kim KM. MMR protein immunohistochemistry and microsatellite instability in gastric cancers. Pathology. 2019; 51:110–3.
Article21. Meyers M, Wagner MW, Hwang HS, Kinsella TJ, Boothman DA. Role of the hMLH1 DNA mismatch repair protein in fluoropyrimidine-mediated cell death and cell cycle responses. Cancer Res. 2001; 61:5193–201.22. Grogg KL, Lohse CM, Pankratz VS, Halling KC, Smyrk TC. Lymphocyte-rich gastric cancer: associations with Epstein-Barr virus, microsatellite instability, histology, and survival. Mod Pathol. 2003; 16:641–51.
Article23. Chiaravalli AM, Feltri M, Bertolini V, Bagnoli E, Furlan D, Cerutti R, et al. Intratumour T cells, their activation status and survival in gastric carcinomas characterised for microsatellite instability and Epstein-Barr virus infection. Virchows Arch. 2006; 448:344–53.
Article24. Onyema OO, Decoster L, Njemini R, Forti LN, Bautmans I, De Waele M, et al. Chemotherapy-induced changes and immunosenescence of CD8+ T-cells in patients with breast cancer. Anticancer Res. 2015; 35:1481–9.25. Mondaca S, Yoon SS, Strong VE, Ku GY, Ilson DH, Greally M, et al. Comment on “Microsatellite instability as a predictive biomarker for adjuvant chemotherapy in gastric cancer”: are we there yet? Ann Surg. 2019; 270:e39–40.26. First tissue-agnostic drug approval issued. Cancer Discov. 2017; 7:656.27. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015; 372:2509–20.28. Sinicrope FA, Shi Q, Allegra CJ, Smyrk TC, Thibodeau SN, Goldberg RM, et al. Association of DNA mismatch repair and mutations in BRAF and KRAS with survival after recurrence in stage III colon cancers: a secondary analysis of 2 randomized clinical trials. JAMA Oncol. 2017; 3:472–80.29. Cho J, Lee J, Bang H, Kim ST, Park SH, An JY, et al. Programmed cell death-ligand 1 expression predicts survival in patients with gastric carcinoma with microsatellite instability. Oncotarget. 2017; 8:13320–8.
Article30. Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015; 21:1350–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gastric Cancer and DNA Mismatch Repair
- Loss of ARID1A Expression in Gastric Cancer: Correlation with Mismatch Repair Deficiency and Clinicopathologic Features
- Hereditary Nonpolyposis Colorectal Cancer
- Microsatellite Instability and Promoter Methylation of hMLH1 in Sporadic Gastric Carcinoma
- Hereditary Colon Cancer: Lynch Syndrome