Cancer Res Treat.
2020 Oct;52(4):1084-1102. 10.4143/crt.2020.093.
β1,4-Galactosyltransferase V Modulates Breast Cancer Stem Cells through Wnt/β-catenin Signaling Pathway
- Affiliations
-
- 1Key Laboratory of Marine Drugs, Chinese Ministry of Education, School of Medicine and Pharmacy, Ocean University of China, Qingdao, China
- 2Laboratory for Marine Drugs and Bioproducts of Qingdao National Laboratory for Marine Science and Technology, Qingdao, China
- 3Open Studio for Druggability Research of Marine Natural Products, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao, China
- KMID: 2507935
- DOI: http://doi.org/10.4143/crt.2020.093
Abstract
- Purpose
Breast cancer stem cells (BCSCs) contribute to the initiation, development, and recurrence of breast carcinomas. β1,4-Galactosyltransferase V (B4GalT5), which catalyzes the addition of galactose to GlcNAcβ1-4Man of N-glycans, is involved in embryogenesis. However, its role in the modulation of BCSCs remains unknown.
Materials and Methods
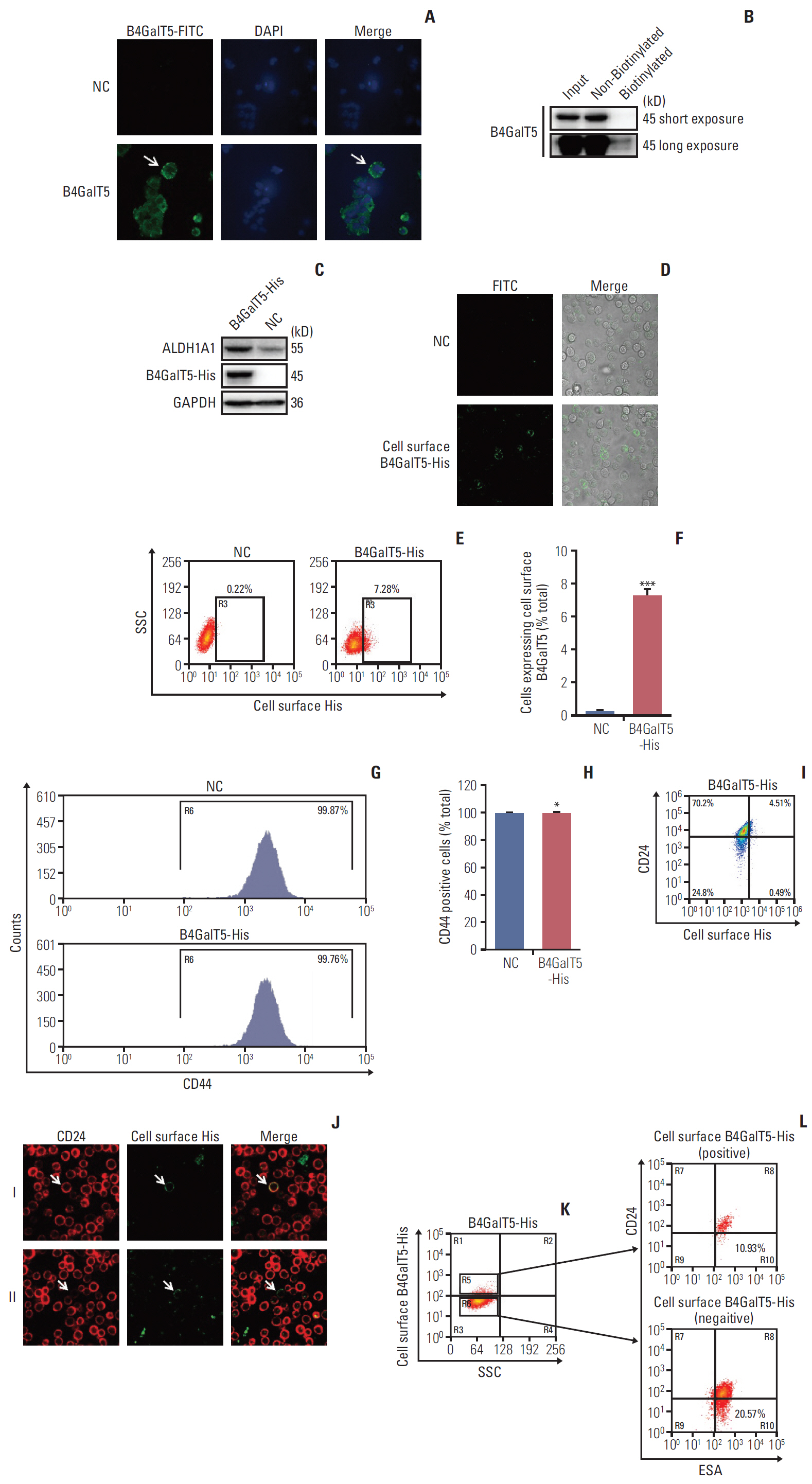
The relationship between B4GalT5 and breast cancer stemness was investigated by online clinical databases and immunohistochemistry analysis. Mammosphere formation, fluorescence-activated cell sorting (FACS), and in-vivo assays were used to evaluate B4GalT5 expression in BCSCs and its effect on BCSCs. B4GalT5 regulation of Wnt/β-catenin signaling was examined by immunofluorescence and Ricinus communis agglutinin I pull-down assays. Cell surface biotinylation and FACS assays were performed to assess the association of cell surface B4GalT5 and BCSCs.
Results
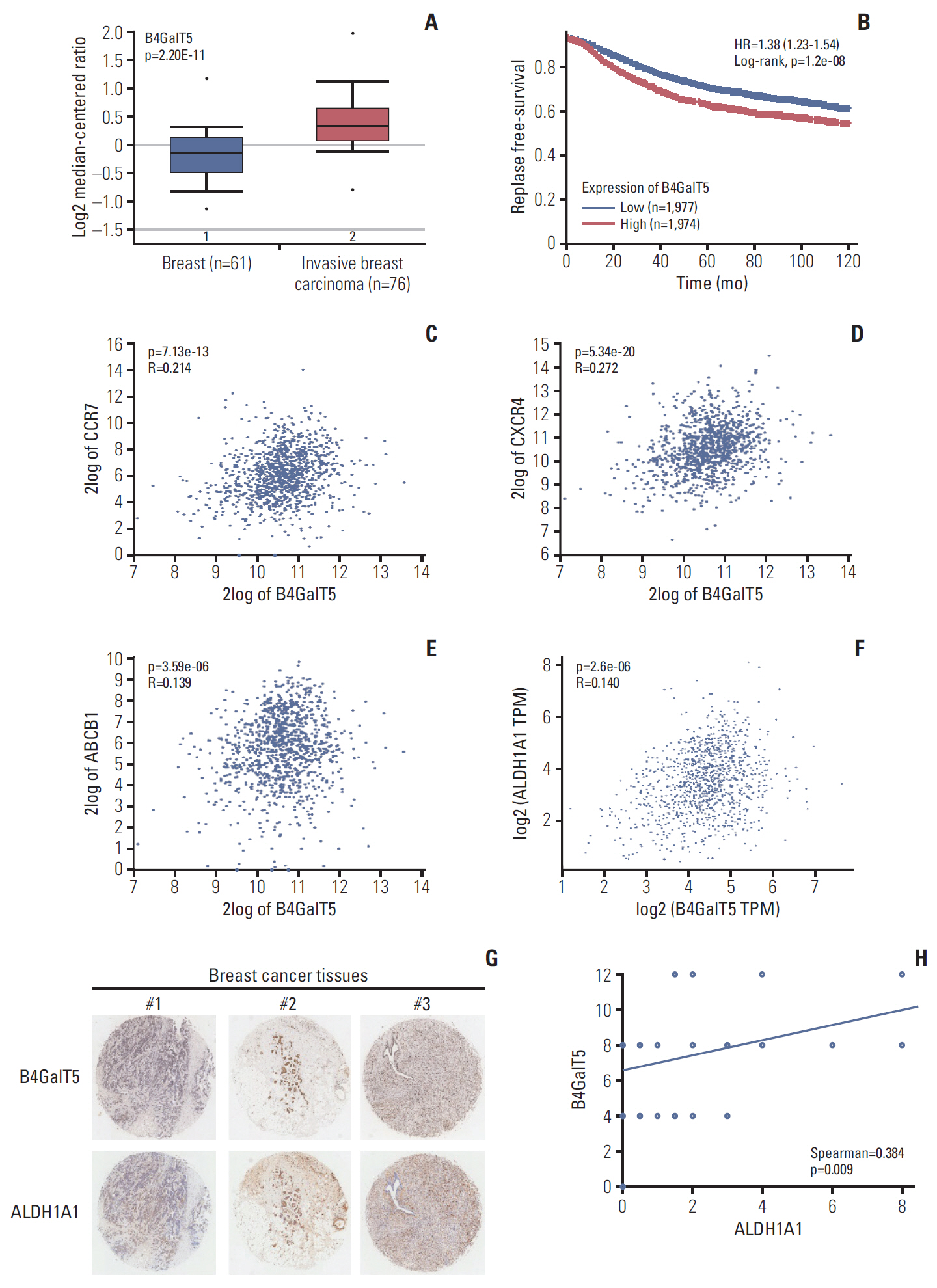
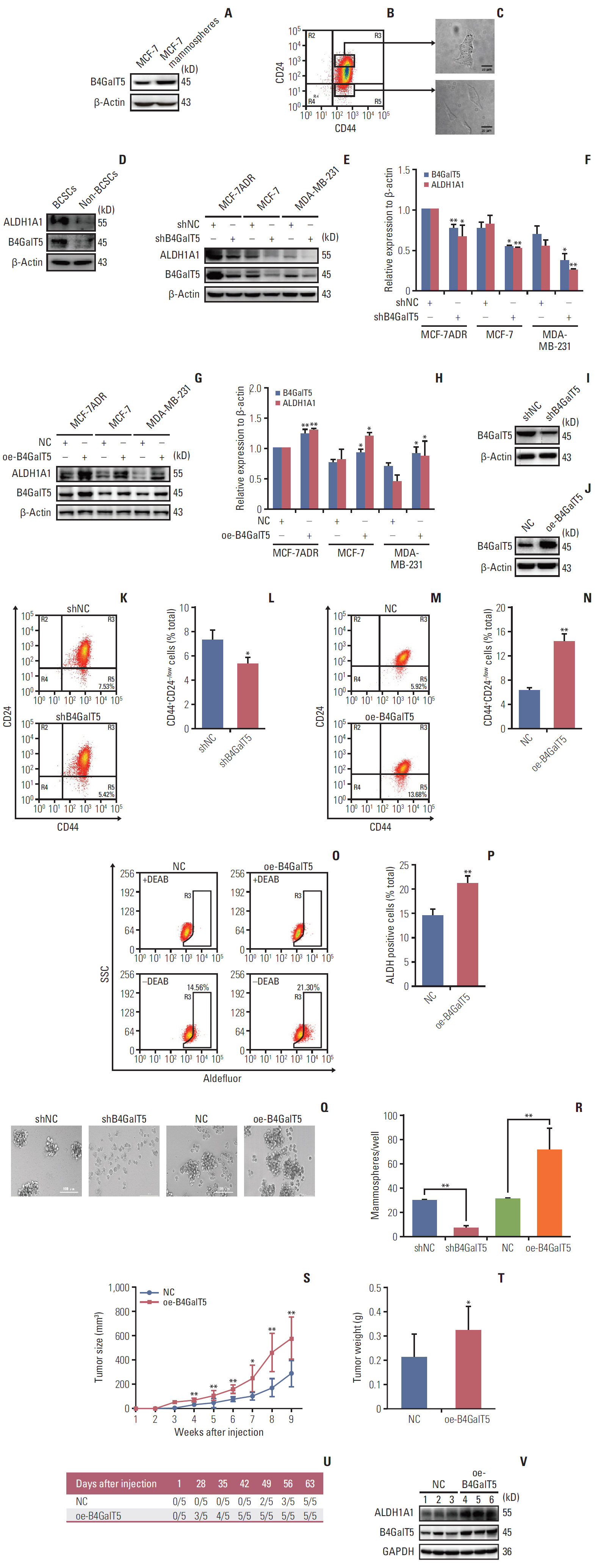
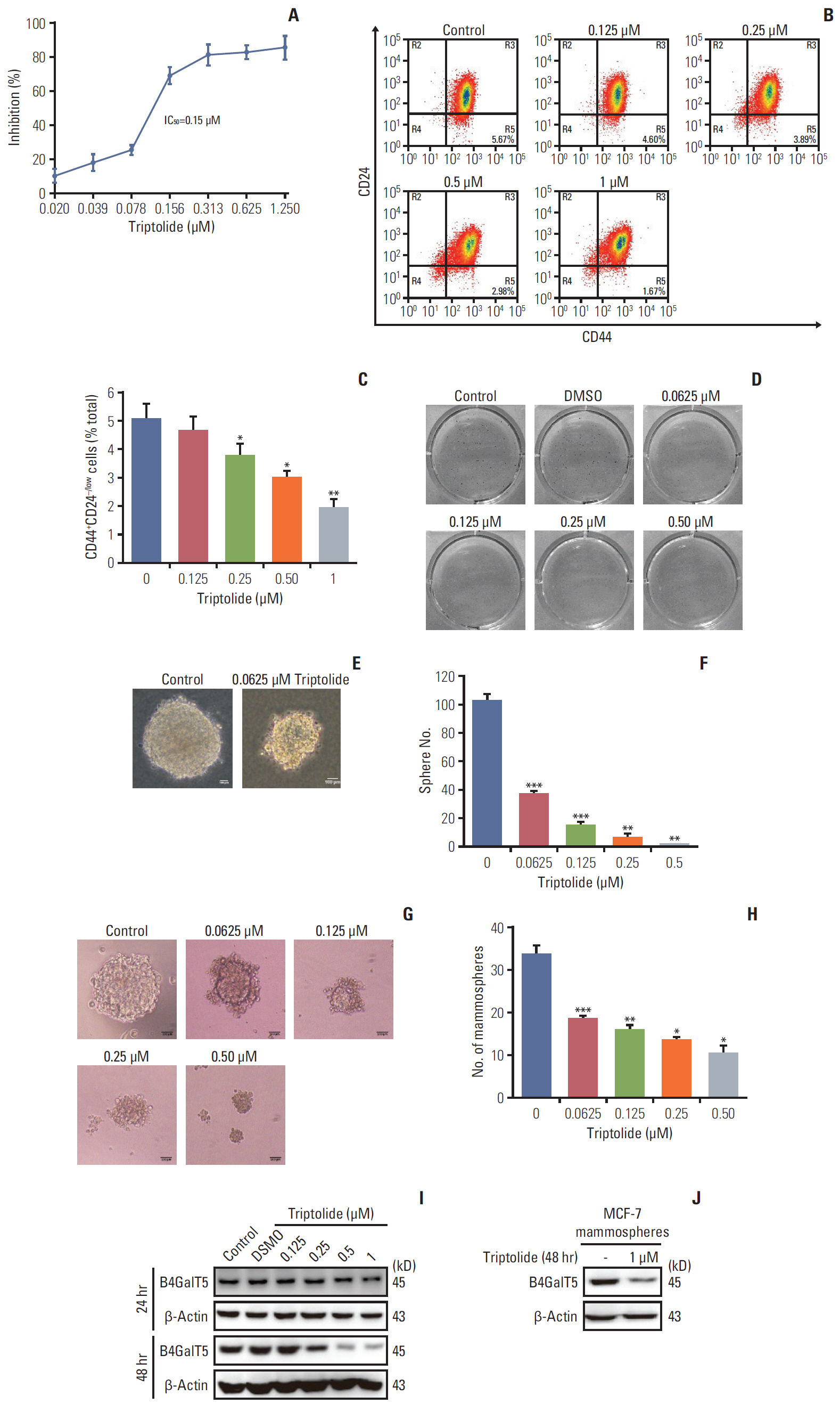
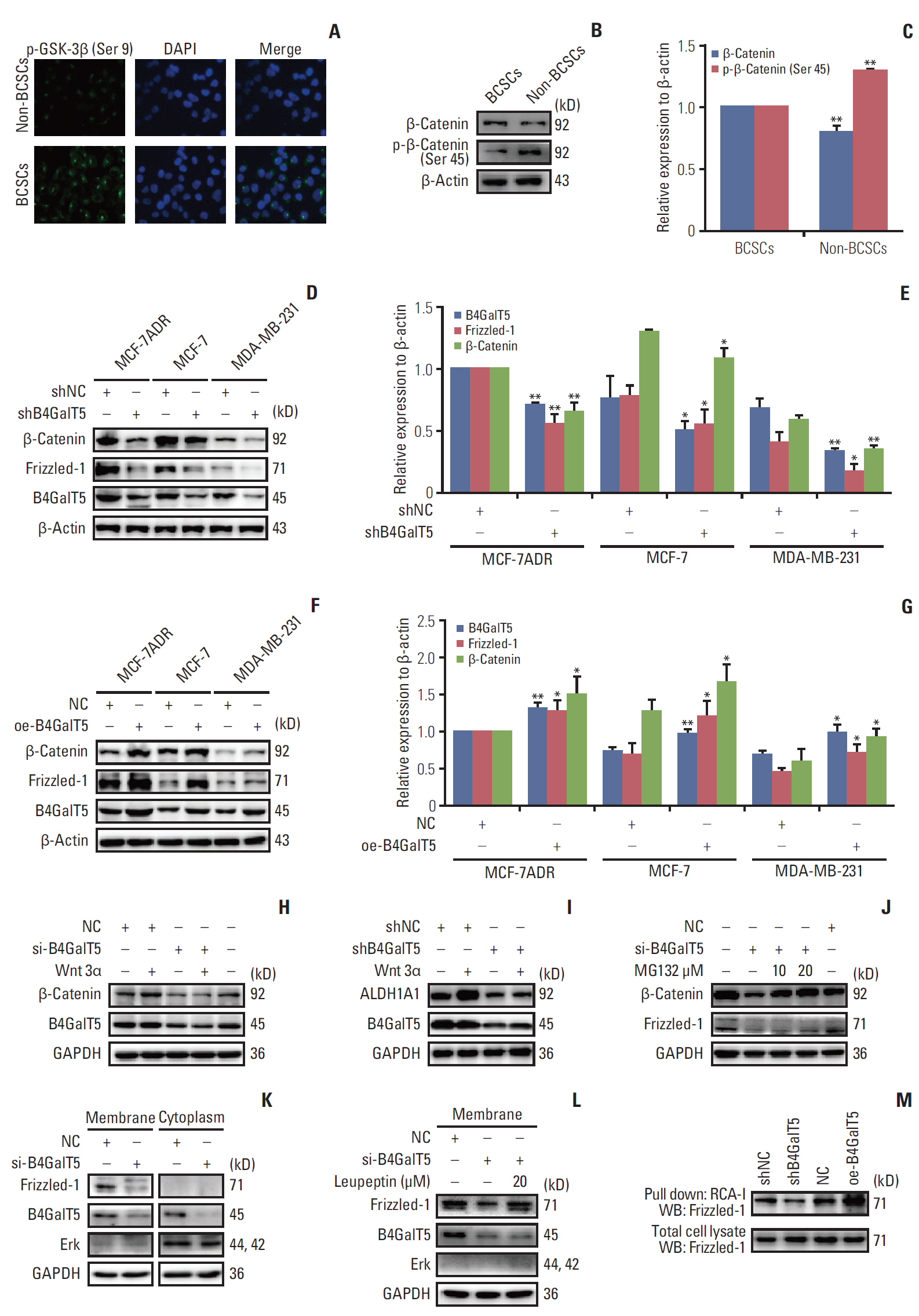
B4GalT5, but not other B4GalTs, was highly correlated with BCSC markers and poor prognosis. B4GalT5 significantly increased the stem cell marker aldehyde dehydrogenase 1A1 (ALDH1A1) and promoted the production of CD44+CD24–/low cells and the formation of mammospheres. Furthermore, B4GalT5 overexpression resulted in dramatic tumor growth in vivo. Mechanistically, B4GalT5 modified and protected Frizzled-1 from degradation via the lysosomal pathway, promoting Wnt/β-catenin signaling which was hyperactivated in BCSCs. B4GalT5, located on the surface of a small subset of breast carcinoma cells, was not responsible for the stemness of BCSCs.
Conclusion
B4GalT5 modulates the stemness of breast cancer through glycosylation modification to stabilize Frizzled-1 and activate Wnt/β-catenin signaling independent of its cell surface location. Our studies highlight a previously unknown role of B4GalT5 in regulating the stemness of breast cancer and provide a potential drug target for anticancer drug development.
Keyword
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012; 10:717–28.
Article3. Ghasemi F, Sarabi PZ, Athari SS, Esmaeilzadeh A. Therapeutics strategies against cancer stem cell in breast cancer. Int J Biochem Cell Biol. 2019; 109:76–81.
Article4. Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005; 5:526–42.
Article5. Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015; 15:540–55.
Article6. Wassler M. β1,4-galactosyltransferases, potential modifiers of stem cell pluripotency and differentiation. In : Bhartiya D, Lenka N, editors. Pluripotent stem cells. Rijeka: InTech;2013. p. 345–72.7. Ma Y, Zhang P, Wang F, Yang J, Yang Z, Qin H. The relationship between early embryo development and tumourigenesis. J Cell Mol Med. 2010; 14:2697–701.
Article8. Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010; 123:725–31.
Article9. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017; 45:W98–W102.
Article10. Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001; 410:50–6.
Article11. Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018; 18:452–64.
Article12. Ciccone V, Terzuoli E, Donnini S, Giachetti A, Morbidelli L, Ziche M. Stemness marker ALDH1A1 promotes tumor angiogenesis via retinoic acid/HIF-1alpha/VEGF signalling in MCF-7 breast cancer cells. J Exp Clin Cancer Res. 2018; 37:311.
Article13. Li Y, Xian M, Yang B, Ying M, He Q. Inhibition of KLF4 by statins reverses adriamycin-induced metastasis and cancer stemness in osteosarcoma cells. Stem Cell Reports. 2017; 8:1617–29.14. Yang A, Qin S, Schulte BA, Ethier SP, Tew KD, Wang GY. MYC inhibition depletes cancer stem-like cells in triple-negative breast cancer. Cancer Res. 2017; 77:6641–50.
Article15. Youakim A, Dubois DH, Shur BD. Localization of the long form of beta-1,4-galactosyltransferase to the plasma membrane and Golgi complex of 3T3 and F9 cells by immunofluorescence confocal microscopy. Proc Natl Acad Sci U S A. 1994; 91:10913–7.
Article16. Lim SK, Lu SY, Kang SA, Tan HJ, Li Z, Adrian Wee ZN, et al. Wnt signaling promotes breast cancer by blocking ITCHmediated degradation of YAP/TAZ transcriptional coactivator WBP2. Cancer Res. 2016; 76:6278–89.
Article17. Chung J, Karkhanis V, Baiocchi RA, Sif S. Protein arginine methyltransferase 5 (PRMT5) promotes survival of lymphoma cells via activation of WNT/beta-catenin and AKT/GSK3beta proliferative signaling. J Biol Chem. 2019; 294:7692–710.18. Rada P, Rojo AI, Offergeld A, Feng GJ, Velasco-Martin JP, Gonzalez-Sancho JM, et al. WNT-3A regulates an Axin1/NRF2 complex that regulates antioxidant metabolism in hepatocytes. Antioxid Redox Signal. 2015; 22:555–71.
Article19. Cooper GM. The cell: a molecular approach. 2nd ed. Sunderland, MA: Sinauer Associates Inc.;2000.20. Sola RJ, Griebenow K. Effects of glycosylation on the stability of protein pharmaceuticals. J Pharm Sci. 2009; 98:1223–45.21. Sasaki N, Manya H, Okubo R, Kobayashi K, Ishida H, Toda T, et al. beta4GalT-II is a key regulator of glycosylation of the proteins involved in neuronal development. Biochem Biophys Res Commun. 2005; 333:131–7.22. Chen WS, Chang HY, Li CP, Liu JM, Huang TS. Tumor beta-1,4-galactosyltransferase IV overexpression is closely associated with colorectal cancer metastasis and poor prognosis. Clin Cancer Res. 2005; 11(24 Pt 1):8615–22.23. Yoshihara T, Satake H, Nishie T, Okino N, Hatta T, Otani H, et al. Lactosylceramide synthases encoded by B4galt5 and 6 genes are pivotal for neuronal generation and myelin formation in mice. PLoS Genet. 2018; 14:e1007545.
Article24. Talhaoui I, Bui C, Oriol R, Mulliert G, Gulberti S, Netter P, et al. Identification of key functional residues in the active site of human {beta}1,4-galactosyltransferase 7: a major enzyme in the glycosaminoglycan synthesis pathway. J Biol Chem. 2010; 285:37342–58.25. Katoh M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review). Int J Oncol. 2017; 51:1357–69.
Article26. Wang H, Zhou T, Peng J, Xu P, Dong N, Chen S, et al. Distinct roles of N-glycosylation at different sites of corin in cell membrane targeting and ectodomain shedding. J Biol Chem. 2015; 290:1654–63.
Article27. Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016; 7:12632.
Article28. Xu Y, Chang R, Xu F, Gao Y, Yang F, Wang C, et al. N-glycosylation at Asn 402 stabilizes N-cadherin and promotes cellcell adhesion of glioma cells. J Cell Biochem. 2017; 118:1423–31.
Article29. Yonamine I, Bamba T, Nirala NK, Jesmin N, KosakowskaCholody T, Nagashima K, et al. Sphingosine kinases and their metabolites modulate endolysosomal trafficking in photoreceptors. J Cell Biol. 2011; 192:557–67.
Article30. Rodeheffer C, Shur BD. Targeted mutations in beta1,4-galactosyltransferase I reveal its multiple cellular functions. Biochim Biophys Acta. 2002; 1573:258–70.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transforming Growth Factor-β Signaling Inhibits the Osteogenic Differentiation of Mesenchymal Stem Cells via Activation of Wnt/β-Catenin Pathway
- Natural Products Targeting Wnt/β-catenin Signaling Pathway
- RNF43 and ZNRF3 in Wnt Signaling - A Master Regulator at the Membrane
- Kinesin Family Member 11 Enhances the Self-Renewal Ability of Breast Cancer Cells by Participating in the Wnt/β-Catenin Pathway
- The crosstalk of Wnt/β-catenin signaling and p53 in acute kidney injury and chronic kidney disease