Ann Lab Med.
2020 Nov;40(6):490-492. 10.3343/alm.2020.40.6.490.
Quality of Ribonucleic Acid Extraction for Real-Time Reverse Transcription-PCR (rRT-PCR) of SARS-CoV-2: Importance of Internal Control Monitoring
- Affiliations
-
- 1Konkuk University School of Medicine, Seoul, Korea
- 2Department of Laboratory Medicine, University of Ulsan, College of Medicine and Asan Medical Center, Seoul, Korea
- KMID: 2507796
- DOI: http://doi.org/10.3343/alm.2020.40.6.490
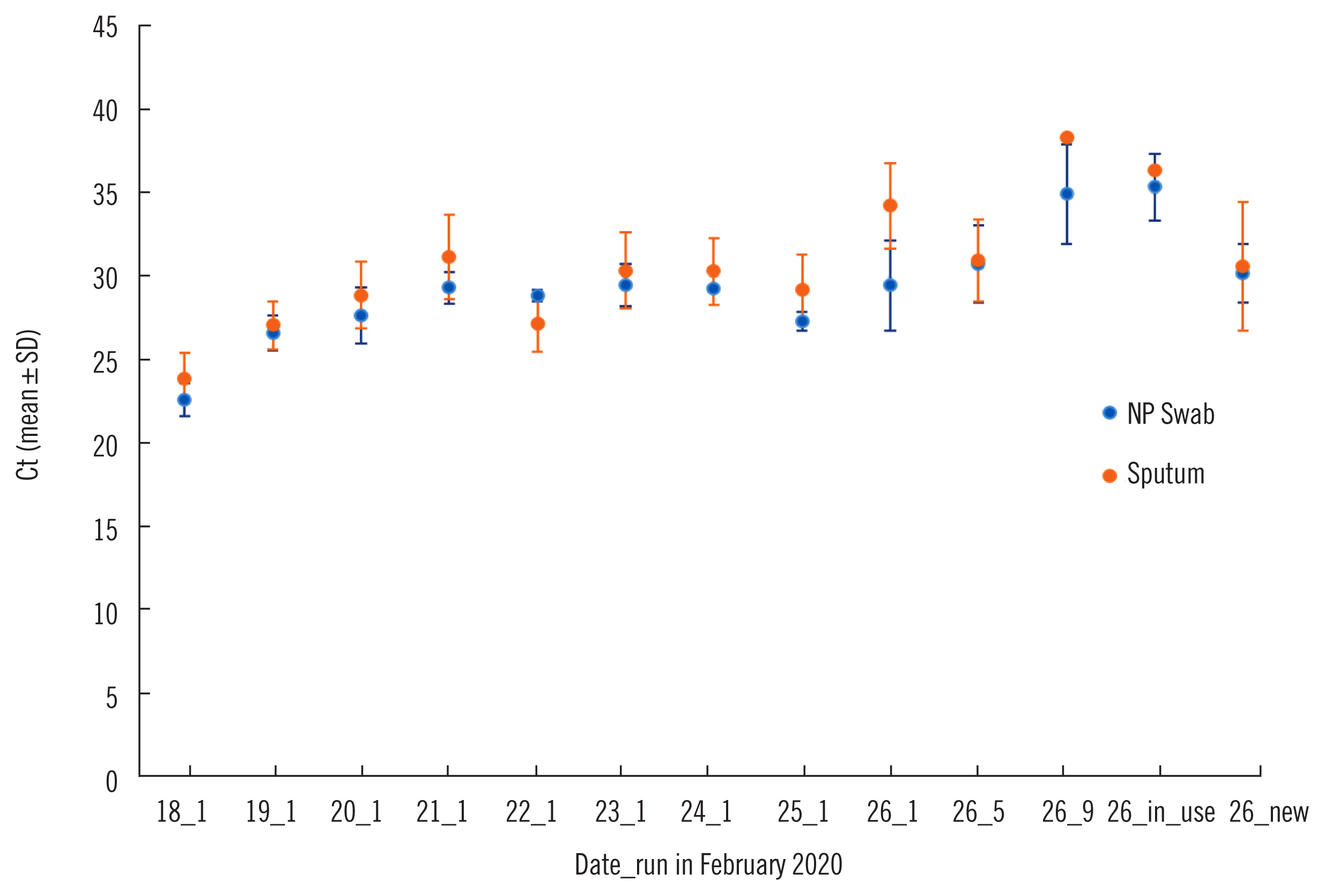
Figure
Cited by 2 articles
-
Surveillance of Coronavirus Disease 2019 (COVID-19) Testing in Clinical Laboratories in Korea
Hee Jae Huh, Ki Ho Hong, Taek Soo Kim, Sang Hoon Song, Kyoung Ho Roh, Hyukmin Lee, Gye Cheol Kwon
Ann Lab Med. 2021;41(2):225-229. doi: 10.3343/alm.2021.41.2.225.Performance Evaluation of the PowerChek SARS-CoV-2, Influenza A & B Multiplex Real-Time PCR Kit in Comparison with the BioFire Respiratory Panel
Tae Yeul Kim, Ji-Youn Kim, Hyang Jin Shim, Sun Ae Yun, Ja-Hyun Jang, Hee Jae Huh, Jong-Won Kim, Nam Yong Lee
Ann Lab Med. 2022;42(4):473-477. doi: 10.3343/alm.2022.42.4.473.
Reference
-
1. World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report–38. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200227-sitrep-38-covid-19.pdf?sfvrsn=9f98940c_4 (Updated on 27 Feb 2020).2. World Health Organization. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases. Interim guidance. https://apps.who.int/iris/handle/10665/331329 (Updated on 2 Mar 2020).3. Korea Centers for Disease Control and Prevention. The 15 new cases of COVID-19 have been confirmed in Korea. https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030&act=view&list_no=366239&tag=&nPage=1 (Updated on 19 Feb 2020).4. Korea Centers for Disease Control and Prevention. The Updates of COVID-19 in Republic of Korea as of 26 February, 2020. https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030&act=view&list_no=366350&tag=&nPage=1 (Updated on 26 Feb 2020).5. Park JS, Choi YS, Yoo CK. Emergency use authorization of in-vitro diagnostics for infectious disease. Public Health Weekly Report. 2017; 10:555–9.6. CLSI. Quantitative molecular methods for infectious disease. Approved guideline. 2nd ed. CLSI MM06-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2010.7. Kim MN, Ko YJ, Seong MW, Kim JS, Shin BM, Sung H. Analytical and clinical validation of six commercial Middle East Respiratory Syndrome coronavirus RNA detection kits based on real-time reverse-transcription PCR. Ann Lab Med. 2016; 36:450–6.
Article8. Kim YJ, Sung H, Ki CS, Hur M. COVID-19 testing in South Korea: current status and the need for faster diagnostics. Ann Lab Med. 2020; 40:349–3509.
Article9. Sung H, Yong D, Ki CS, Kim JS, Seong MW, Lee H, et al. Comparative evaluation of three homogenization methods for isolating Middle East Respiratory Syndrome coronavirus nucleic acids from sputum samples for real-time reverse transcription PCR. Ann Lab Med. 2016; 36:457–62.
Article10. Lee MK, Kim S, Kim MN, Kweon OJ, Lim YK, Ki CS, et al. Survey of clinical laboratory practices for 2015 Middle East Respiratory Syndrome coronavirus outbreak in the Republic of Korea. Ann Lab Med. 2016; 36:154–61.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Understandings and Prospects of Laboratory Diagnosis of SARS-CoV-2
- Evaluation of the AdvanSure One-Stop COVID-19 Plus Kit for SARS-CoV-2 Detection Using a Streamlined RNA Extraction Method
- Comparison of Rapid Antigen Test and Real-Time Reverse Transcription PCR for the Detection of Influenza B Virus
- Laboratory Diagnosis of COVID-19 in Korea
- Evaluation of Three Multiplex Realtime Reverse Transcription PCR Assays for Simultaneous Detection of SARS-CoV-2, Influenza A/B, and Respiratory Syncytial Virus in Nasopharyngeal Swabs