Clin Endosc.
2020 Sep;53(5):575-582. 10.5946/ce.2019.150.
Reasons for Diagnostic Failure in Forty-Five Consecutive Mucosal Cutting Biopsy Examinations of Gastric Subepithelial Tumors
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Kobe University Graduate School of Medicine, Kobe, Japan
- 2Department of Endoscopy, Kobe University Hospital, Kobe, Japan
- KMID: 2507592
- DOI: http://doi.org/10.5946/ce.2019.150
Abstract
- Background/Aims
Mucosal cutting biopsy (MCB) is useful for the histopathological diagnosis of gastric subepithelial tumors (SETs). However, there is little information on cases in which MCB did not establish a diagnosis. In the current study, we aimed to investigate the characteristics of cases in which MCB was unsuccessful.
Methods
Cases in which MCB was used to histopathologically diagnose gastric SETs at Kobe University Hospital between August 2012 and October 2018 were retrospectively reviewed.
Results
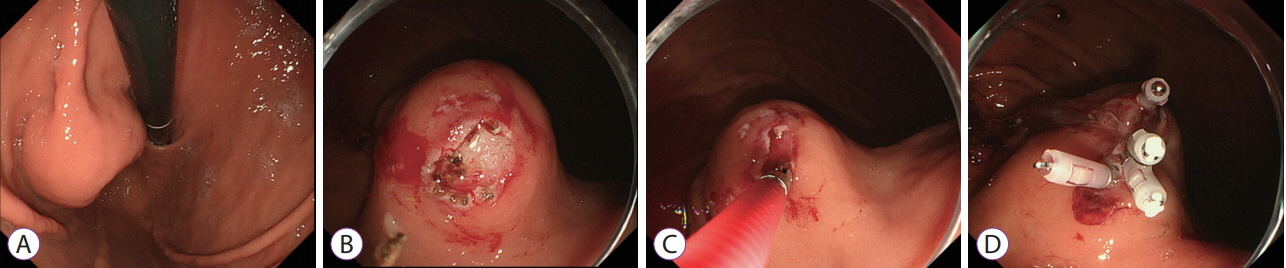
Forty-five cases in which MCB was used to diagnose 43 gastric SETs in 43 patients were analyzed. The median tumor size was 20 mm (range, 8–50 mm). Pathological examinations resulted in definitive and suspected diagnoses and no diagnosis in 29 (gastrointestinal stromal tumor: n=17, leiomyoma: n=7, aberrant pancreas: n=3, others: n=2), 6, and 10 cases, respectively. Failure to expose the tumor according to retrospective examinations of endoscopic images was significantly associated with no diagnosis. Other possible explanations included a less elevated tumor, biopsy of the surrounding field instead of the tumor due to the mobility, and poor endoscope maneuverability due to the tumor being close to the cardia.
Conclusions
Clear exposure of gastric SETs during MCB may improve the diagnostic rate of such examinations.
Figure
Cited by 1 articles
-
Mucosal Incision-Assisted Endoscopic Biopsy as an Alternative to Endoscopic Ultrasound-Guided Fine-Needle Aspiration/Biopsy for Gastric Subepithelial Tumor
Cheol Woong Choi, Joo Ha Hwang
Clin Endosc. 2020;53(5):505-507. doi: 10.5946/ce.2020.187.
Reference
-
1. Hwang JH, Kimmey MB. The incidental upper gastrointestinal subepithelial mass. Gastroenterology. 2004; 126:301–307.
Article2. Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991; 5:20–23.3. Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005; 37:635–645.
Article4. Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010; 71:913–919.
Article5. Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000; 15:1293–1301.6. Japan Society of Clinical Oncology. Japanese Gastric Cancer Association, Japanese Study Group on GIST. GIST therapeutic guidelines. Tokyo: Kanehara & Co;2008.7. Yoshida S, Yamashita K, Yokozawa M, et al. Diagnostic findings of ultrasound-guided fine-needle aspiration cytology for gastrointestinal stromal tumors: proposal of a combined cytology with newly defined features and histology diagnosis. Pathol Int. 2009; 59:712–719.
Article8. Ando N, Goto H, Niwa Y, et al. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002; 55:37–43.
Article9. Ecka RS, Sharma M. Rapid on-site evaluation of EUS-FNA by cytopathologist: an experience of a tertiary hospital. Diagn Cytopathol. 2013; 41:1075–1080.
Article10. Ihara E, Matsuzaka H, Honda K, et al. Mucosal-incision assisted biopsy for suspected gastric gastrointestinal stromal tumors. World J Gastrointest Endosc. 2013; 5:191–196.
Article11. Kataoka M, Kawai T, Yagi K, et al. Mucosal cutting biopsy technique for histological diagnosis of suspected gastrointestinal stromal tumors of the stomach. Dig Endosc. 2013; 25:274–280.
Article12. Ikehara H, Li Z, Watari J, et al. Histological diagnosis of gastric submucosal tumors: a pilot study of endoscopic ultrasonography-guided fine-needle aspiration biopsy vs mucosal cutting biopsy. World J Gastrointest Endosc. 2015; 7:1142–1149.
Article13. Liu YM, Yang XJ. Endoscopic ultrasound-guided cutting of holes and deep biopsy for diagnosis of gastric infiltrative tumors and gastrointestinal submucosal tumors using a novel vertical diathermic loop. World J Gastroenterol. 2017; 23:2795–2801.
Article14. Lee CK, Chung IK, Lee SH, et al. Endoscopic partial resection with the unroofing technique for reliable tissue diagnosis of upper GI subepithelial tumors originating from the muscularis propria on EUS (with video). Gastrointest Endosc. 2010; 71:188–194.
Article15. Kobara H, Mori H, Fujihara S, et al. Bloc biopsy by using submucosal endoscopy with a mucosal flap method for gastric subepithelial tumor tissue sampling (with video). Gastrointest Endosc. 2013; 77:141–145.
Article16. Ito H, Inoue H, Ryozawa S, et al. Fine-needle aspiration biopsy and endoscopic ultrasound for pretreatment pathological diagnosis of gastric gastrointestinal stromal tumors. Gastroenterol Res Pract. 2012; 2012:139083.
Article17. Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007; 13:2077–2082.
Article18. Okada K, Maruyama K, Nagase H, et al. [A case of gastrointestinal stromal tumor of the stomach with rapid growth in a short term]. Gan To Kagaku Ryoho. 2008; 35:2080–2082.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic Management of Gastric Subepithelial Tumor
- Advancements in the Diagnosis of Gastric Subepithelial Tumors
- Mucosal Incision and Forceps Biopsy for Reliable Tissue Sampling of Gastric Subepithelial Tumors
- Gastric Subepithelial Tumor Diagnosed by Transabdominal Ultrasonography
- Common Gastric Subepithelial Tumors in Koreans