Ann Pediatr Endocrinol Metab.
2020 Sep;25(3):182-186. 10.6065/apem.1938154.077.
Effectiveness of growth hormone therapy in children with Noonan syndrome
- Affiliations
-
- 1Department of Pediatrics, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea
- 2Department of Pediatrics, Gyeongsang National University Changwon Hospital, Changwon, Korea
- KMID: 2507561
- DOI: http://doi.org/10.6065/apem.1938154.077
Abstract
- Purpose
Recombinant human growth hormone (rhGH) has been used to improve growth in children with Noonan syndrome (NS). This study aimed to investigate the efficacy of rhGH therapy in Korean children with NS.
Methods
Seventeen prepubertal children (10 boys, 7 girls) with NS who received rhGH therapy for at least 3 years between 2008 and 2017 were included. To compare the response, age- and sex-matched children with GH deficiency (GHD; n=31) were included. Height and growth velocity before and during treatment were analyzed.
Results
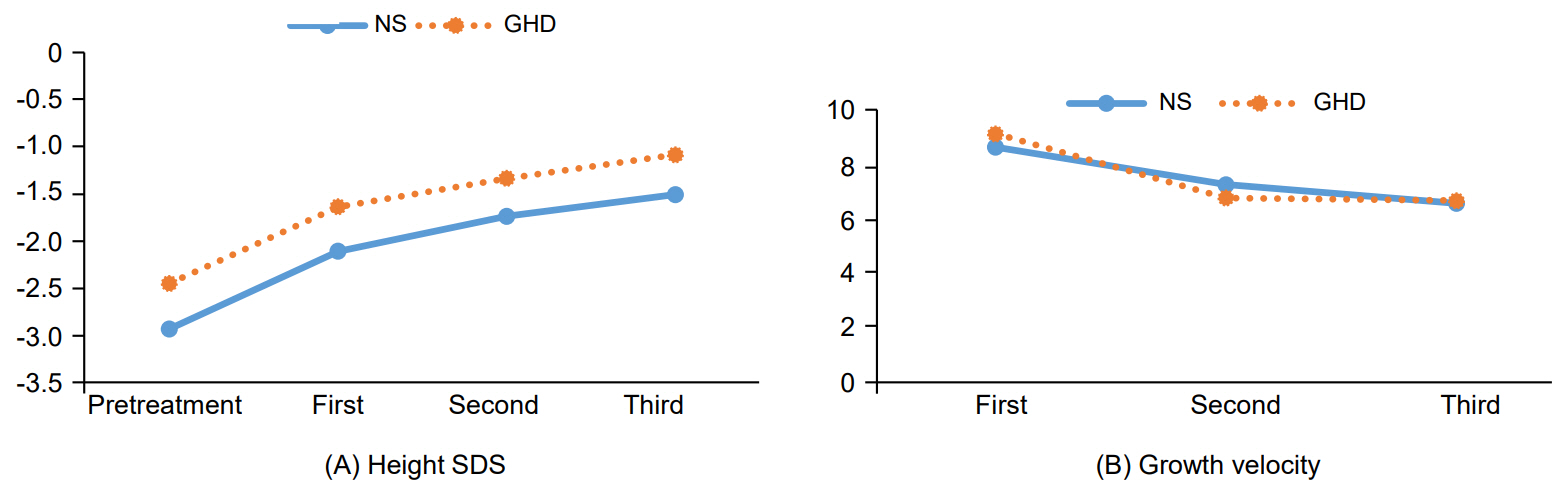
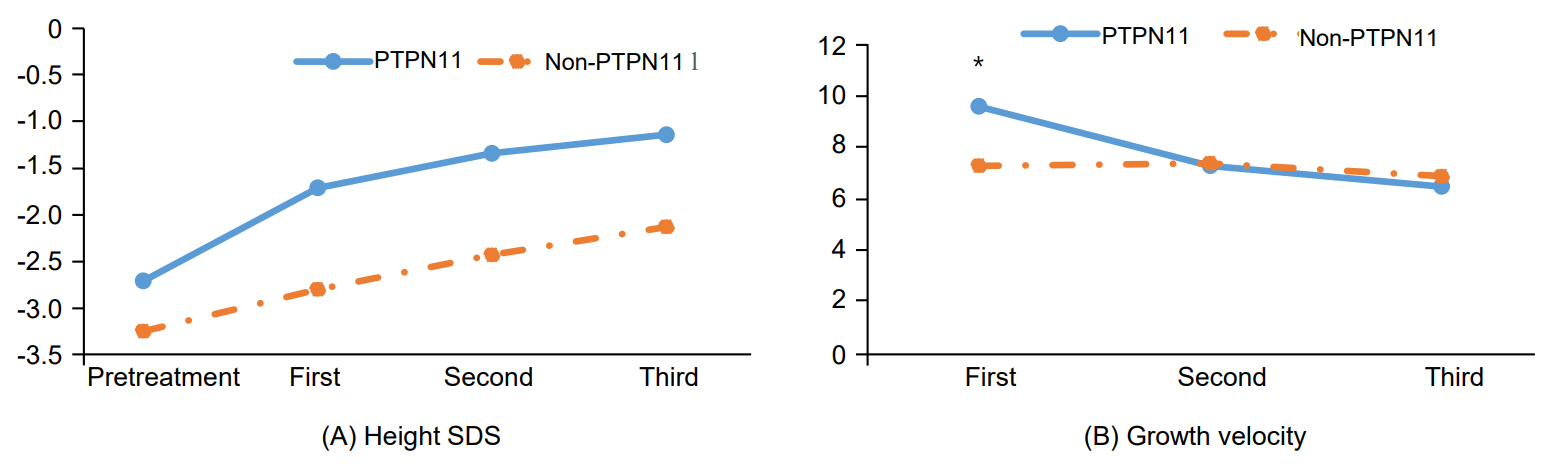
The mean age of NS patients was 6.34±2.32 years. After treatment, the height standard deviation score (SDS) increased from -2.93±0.81 to -1.51±1.00 in patients with NS and from -2.45±0.42 to -1.09±0.47 in patients with GHD. There were no significant differences in growth velocity or change in height SDS between patients with NS and GHD. Growth velocity in the first year of treatment was higher in patients with PTPN11 mutations than those without PTPN11 mutations, but the change in height SDS was not significantly different between those 2 groups.
Conclusion
rhGH therapy can increase linear growth in prepubertal children with NS. The growth response between patients with NS and patients with GHD was not significantly different. Furthermore, we observed that lower doses of growth hormone have a similar effect on height compared to previous studies in patients with NS. Our study indicates that rhGH treatment is useful for growth promotion.
Keyword
Figure
Cited by 1 articles
-
Approach to Short Stature in Children and Adolescent
Hyo-Kyoung Nam
Ewha Med J. 2021;44(4):111-116. doi: 10.12771/emj.2021.44.4.111.
Reference
-
References
1. Roberts AE, Allanson JE, Tartaglia M, Gelb BD. Noonan syndrome. Lancet. 2013; 381:333–42.
Article2. Binder G. Noonan syndrome, the Ras-MAPK signalling pathway and short stature. Horm Res. 2009; 71 Suppl 2:64–70.
Article3. Noordam K. Expanding the genetic spectrum of Noonan syndrome. Horm Res. 2007; 68 Suppl 5:24–7.
Article4. Romano AA, Allanson JE, Dahlgren J, Gelb BD, Hall B, Pierpont ME, et al. Noonan syndrome: clinical features, diagnosis, and management guidelines. Pediatrics. 2010; 126:746–59.
Article5. Padidela R, Camacho-Hubner C, Attie KM, Savage MO. Abnormal growth in noonan syndrome: genetic and endocrine features and optimal treatment. Horm Res. 2008; 70:129–36.
Article6. Yart A, Edouard T. Noonan syndrome: an update on growth and development. Curr Opin Endocrinol Diabetes Obes. 2018; 25:67–73.
Article7. van der Burgt I. Noonan syndrome. Orphanet J Rare Dis. 2007; 2:4.
Article8. Hyun SE, Lee BC, Suh BK, Chung SC, Ko CW, Kim HS, et al. Reference values for serum levels of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in Korean children and adolescents. Clin Biochem. 2012; 45:16–21.
Article9. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018; 61:135–49.
Article10. Greulich WW, Pyle SI. R adiologic atlas of skeletal development of the hand and wrist. 2nd ed. Standford: Stanford University Press;1959. p. 53.11. Wheeler MD. Physical changes of puberty. Endocrinol Metab Clin North Am. 1991; 20:1–14.
Article12. Jo KJ, Kim YM, Yoon JY, Lee YJ, Han YM, Yoo HW, et al. Comparison of effectiveness of growth hormone therapy according to disease-causing genes in children with Noonan syndrome. Korean J Pediatr. 2019; 62:274–80.
Article13. Jeong I, Kang E, Cho JH, Kim GH, Lee BH, Choi JH, et al. Long-term efficacy of recombinant human growth hormone therapy in short-statured patients with Noonan syndrome. Ann Pediatr Endocrinol Metab. 2016; 21:26–30.
Article14. Zavras N, Meazza C, Pilotta A, Gertosio C, Pagani S, Tinelli C, et al. Five-year response to growth hormone in children with Noonan syndrome and growth hormone deficiency. Ital J Pediatr. 2015; 41:71.
Article15. Choi JH, Lee BH, Jung CW, Kim YM, Jin HY, Kim JM, et al. Response to growth hormone therapy in children with Noonan syndrome: correlation with or without PTPN11 gene mutation. Horm Res Paediatr. 2012; 77:388–93.
Article16. Romano AA, Dana K, Bakker B, Davis DA, Hunold JJ, Jacobs J, et al. Growth response, near-adult height, and patterns of growth and puberty in patients with noonan syndrome treated with growth hormone. J Clin Endocrinol Metab. 2009; 94:2338–44.
Article17. Osio D, Dahlgren J, Wikland KA, Westphal O. Improved final height with long-term growth hormone treatment in Noonan syndrome. Acta Paediatr. 2005; 94:1232–7.
Article18. De Schepper J, Otten BJ, Francois I, Bourguignon JP, Craen M, Van der Burgt I, et al. Growth hormone therapy in prepubertal children with Noonan syndrome: first year growth response and comparison with Turner syndrome. Acta Paediatr. 1997; 86:943–6.
Article19. Giacomozzi C, Deodati A, Shaikh MG, Ahmed SF, Cianfarani S. The impact of growth hormone therapy on adult height in noonan syndrome: a systematic review. Horm Res Paediatr. 2015; 83:167–76.
Article20. Noonan JA, Kappelgaard AM. The efficacy and safety of growth hormone therapy in children with noonan syndrome: a review of the evidence. Horm Res Paediatr. 2015; 83:157–66.
Article21. Binder G, Neuer K, Ranke MB, Wittekindt NE. PTPN11 mutations are associated with mild growth hormone resistance in individuals with Noonan syndrome. J Clin Endocrinol Metab. 2005; 90:5377–81.22. Malaquias AC, Noronha RM, Souza TTO, Homma TK, Funari MFA, Yamamoto GL, et al. Impact of growth hormone therapy on adult height in patients with PTPN11 mutations related to Noonan syndrome. Horm Res Paediatr. 2019; 91:252–61.
Article23. Noordam C, Peer PG, Francois I, De Schepper J, van den Burgt I, Otten BJ. Long-term GH treatment improves adult height in children with Noonan syndrome with and without mutations in protein tyrosine phosphatase, non-receptor-type 11. Eur J Endocrinol. 2008; 159:203–8.
Article24. Lee S, Kwon AR, Chae HW, Kim HS. Efficacy of growth hormone treatment in patients with Noonan syndrome and growth hormone deficiency. J Korean Soc Pediatr Endocrinol. 2011; 16:100–5.
Article25. Ozono K, Ogata T, Horikawa R, Matsubara Y, Ogawa Y, Nishijima K, et al. Efficacy and safety of two doses of Norditropin((R)) (somatropin) in short stature due to Noonan syndrome: a 2-year randomized, double-blind, multicenter trial in Japanese patients. Endocr J. 2018; 65:159–74.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Characteristics and Growth Responses to GH Therapy in Children with Noonan Syndrome
- Correlation between genetic heterogeneity and variability for response to growth hormone in Noonan syndrome
- A case of Noonan syndrome diagnosed using the facial recognition software (FACE2GENE)
- Growth hormone therapy in patients with Noonan syndrome
- Growth Hormone Therapy in Short Stature Children