Yeungnam Univ J Med.
2020 Jul;37(3):169-178. 10.12701/yujm.2020.00374.
Current aspects and prospects of glass ionomer cements for clinical dentistry
- Affiliations
-
- 1Department of Dentistry, Yeungnam University College of Medicine, Daegu, Korea
- 2Department of Dentistry, Yeungnam University Hospital, Daegu, Korea
- KMID: 2506515
- DOI: http://doi.org/10.12701/yujm.2020.00374
Abstract
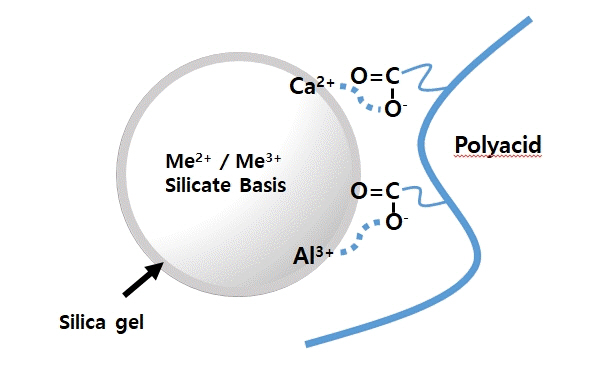
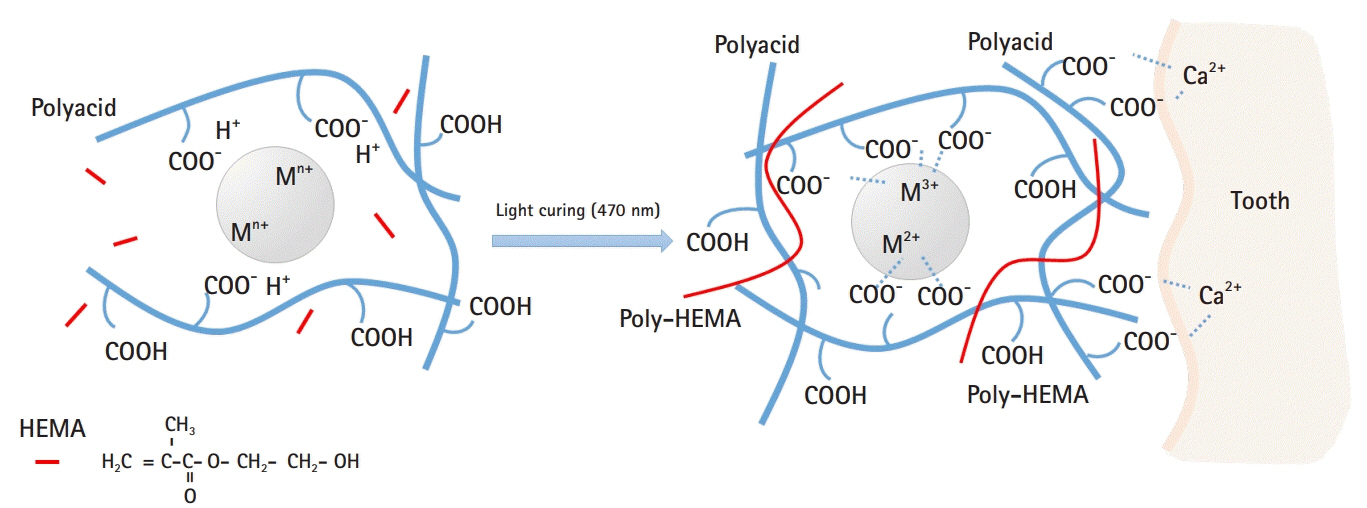
- Glass ionomer cement (GIC) is a tailor-made material that is used as a filling material in dentistry. GIC is cured by an acid-base reaction consisting of a glass filler and ionic polymers. When the glass filler and ionic polymers are mixed, ionic bonds of the material itself are formed. In addition, the extra polymer anion reacts with calcium in enamel or dentin to increase adhesion to the tooth tissue. GICs are widely used as adhesives for artificial crowns or orthodontic brackets, and are also used as tooth repair material, cavity liner, and filling materials. In this review, the current status of GIC research and development and its prospects for the future have been discussed in detail.
Keyword
Figure
Reference
-
References
1. Mount GJ. An atlas of glass-ionomer cements: a clinician’s guide. 2nd ed. Martin Dunitz: London;2002.2. Sidhu SK, Nicholson JW. A review of glass-ionomer cements for clinical dentistry. J Funct Biomater. 2016; 7:16.
Article3. Khoroushi M, Keshani F. A review of glass-ionomers: from conventional glass-ionomer to bioactive glass-ionomer. Dent Res J (Isfahan). 2013; 10:411–20.4. Sidhu SK, Watson TF. Resin-modified glass ionomer materials: a status report for the American Journal of Dentistry. Am J Dent. 1995; 8:59–67.5. Wilson AD. Resin-modified glass-ionomer cements. Int J Prosthodont. 1990; 3:425–9.6. Nicholson JW. Polyacid-modified composite resins (“compomers”) and their use in clinical dentistry. Dent Mater. 2007; 23:615–22.
Article7. Najeeb S, Khurshid Z, Zafar MS, Khan AS, Zohaib S, Marti JM, et al. Modifications in glass ionomer cements: nano-sized fillers and bioactive nanoceramics. Int J Mol Sci. 2016; 17:1134.
Article8. De Caluwe T, Vercruysse CW, Ladik I, Convents R, Declercq H, Martens LC, et al. Addition of bioactive glass to glass ionomer cements: effect on the physico-chemical properties and biocompatibility. Dent Mater. 2017; 33:e186–203.9. Berg JH, Croll TP. Glass ionomer restorative cement systems: an update. Pediatr Dent. 2015; 37:116–24.10. Wilson AD, Kent BE. A new translucent cement for dentistry: the glass ionomer cement. Br Dent J. 1972; 132:133–5.
Article11. Williams JA, Billington RW, Pearson GJ. The comparative strengths of commercial glass-ionomer cements with and without metal additions. Br Dent J. 1992; 172:279–82.
Article12. Cho SY, Cheng AC. A review of glass ionomer restorations in the primary dentition. J Can Dent Assoc. 1999; 65:491–5.13. Frankenberger R, Sindel J, Kramer N. Viscous glass-ionomer cements: a new alternative to amalgam in the primary dentition? Quintessence Int. 1997; 28:667–76.14. Berg JH. The continuum of restorative materials in pediatric dentistry: a review for the clinician. Pediatr Dent. 1998; 20:93–100.15. Nicholson JW, Czarnecka B, Limanowska-Shaw H. The long-term interaction of dental cements with lactic acid solutions. J Mater Sci Mater Med. 1999; 10:449–52.16. Mathis RS, Ferracane JL. Properties of a glass-ionomer/resin-composite hybrid material. Dent Mater. 1989; 5:355–8.
Article17. Burgess J, Norling B, Summitt J. Resin ionomer restorative materials: the new generation. J Esthet Dent. 1994; 6:207–15.
Article18. Xie D, Brantley WA, Culbertson BM, Wang G. Mechanical properties and microstructures of glass-ionomer cements. Dent Mater. 2000; 16:129–38.
Article19. Nagaraja UP, Kishore G. Glass ionomer cement: The different generations. Trends Biomater Artif Organs. 2005; 18:158–65.20. Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials: fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007; 23:343–62.21. Robertello FJ, Coffey JP, Lynde TA, King P. Fluoride release of glass ionomer-based luting cements in vitro. J Prosthet Dent. 1999; 82:172–6.
Article22. Tjandrawinata R, Irie M, Suzuki K. Marginal gap formation and fluoride release of resin-modified glass-ionomer cement: effect of silanized spherical silica filler addition. Dent Mater J. 2004; 23:305–13.
Article23. Musa A, Pearson GJ, Gelbier M. In vitro investigation of fluoride ion release from four resin-modified glass polyalkenoate cements. Biomaterials. 1996; 17:1019–23.
Article24. Momoi Y, McCabe JF. Fluoride release from light-activated glass ionomer restorative cements. Dent Mater. 1993; 9:151–4.
Article25. Attar N, Turgut MD. Fluoride release and uptake capacities of fluoride-releasing restorative materials. Oper Dent. 2003; 28:395–402.26. Karantakis P, Helvatjoglou-Antoniades M, Theodoridou-Pahini S, Papadogiannis Y. Fluoride release from three glass ionomers, a compomer, and a composite resin in water, artificial saliva, and lactic acid. Oper Dent. 2000; 25:20–5.27. Hayacibara MF, Ambrozano GM, Cury JA. Simultaneous release of fluoride and aluminum from dental materials in various immersion media. Oper Dent. 2004; 29:16–22.28. Yap AU, Tham SY, Zhu LY, Lee HK. Short-term fluoride release from various aesthetic restorative materials. Oper Dent. 2002; 27:259–65.29. Gao W, Smales RJ, Gale MS. Fluoride release/uptake from newer glass-ionomer cements used with the ART approach. Am J Dent. 2000; 13:201–4.30. Yli-Urpo H, Vallittu PK, Narhi TO, Forsback AP, Vakiparta M. Release of silica, calcium, phosphorus, and fluoride from glass ionomer cement containing bioactive glass. J Biomater Appl. 2004; 19:5–20.
Article31. Osinaga PW, Grande RH, Ballester RY, Simionato MR, Delgado Rodrigues CR, Muench A. Zinc sulfate addition to glass-ionomer-based cements: influence on physical and antibacterial properties, zinc and fluoride release. Dent Mater. 2003; 19:212–7.
Article32. Mazzaoui SA, Burrow MF, Tyas MJ, Dashper SG, Eakins D, Reynolds EC. Incorporation of casein phosphopeptide-amorphous calcium phosphate into a glass-ionomer cement. J Dent Res. 2003; 82:914–8.
Article33. Lucas ME, Arita K, Nishino M. Toughness, bonding and fluoride-release properties of hydroxyapatite-added glass ionomer cement. Biomaterials. 2003; 24:3787–94.
Article34. Mousavinasab SM, Meyers I. Fluoride release by glass ionomer cements, compomer and giomer. Dent Res J (Isfahan). 2009; 6:75–81.35. Palmer G, Anstice HM, Pearson GJ. The effect of curing regime on the release of hydroxyethyl methacrylate (HEMA) from resin-modified glass-ionomer cements. J Dent. 1999; 27:303–11.
Article36. Hamid A, Hume WR. Diffusion of resin monomers through human carious dentin in vitro. Endod Dent Traumatol. 1997; 13:1–5.
Article37. Kan KC, Messer LB, Messer HH. Variability in cytotoxicity and fluoride release of resin-modified glass-ionomer cements. J Dent Res. 1997; 76:1502–7.
Article38. McLean JW, Nicholson JW, Wilson AD. Proposed nomenclature for glass-ionomer dental cements and related materials. Quintessence Int. 1994; 25:587–9.39. Meyer JM, Cattani-Lorente MA, Dupuis V. Compomers: between glass-ionomer cements and composites. Biomaterials. 1998; 19:529–39.
Article40. Eliades G, Kakaboura A, Palaghias G. Acid-base reaction and fluoride release profiles in visible light-cured polyacid-modified composite restoratives (compomers). Dent Mater. 1998; 14:57–63.
Article41. Ruse ND. What is a “compomer”? J Can Dent Assoc. 1999; 65:500–4.42. Young AM, Rafeeka SA, Howlett JA. FTIR investigation of monomer polymerisation and polyacid neutralisation kinetics and mechanisms in various aesthetic dental restorative materials. Biomaterials. 2004; 25:823–33.
Article43. Nicholson JW, Alsarheed M. Changes on storage of polyacid-modified composite resins. J Oral Rehabil. 1998; 25:616–20.
Article44. Dahl JE, Li J, Ruyter IE. Long-term water uptake of compomers and its effect on mechanical properties. J Dent Res. 1998; 77(2 Suppl):657 (abstract 207).45. Adusei GO, Deb S, Nicholson JW. A preliminary study of experimental polyacid-modified composite resins ('compomers') containing vinyl phosphonic acid. Dent Mater. 2005; 21:491–7.
Article46. Mendonca JS, Souza MH Jr, Carvalho RM. Effect of storage time on microtensile strength of polyacid-modified resin composites. Dent Mater. 2003; 19:308–12.
Article47. Bayindir YZ, Yildiz M. Surface hardness properties of resin-modified glass ionomer cements and polyacid-modified composite resins. J Contemp Dent Pract. 2004; 5:42–9.
Article48. Loguercio AD, Reis A, Barbosa AN, Roulet JF. Five-year double-blind randomized clinical evaluation of a resin-modified glass ionomer and a polyacid-modified resin in noncarious cervical lesions. J Adhes Dent. 2003; 5:323–32.49. Ermis RB. Two-year clinical evaluation of four polyacid-modified resin composites and a resin-modified glass-ionomer cement in Class V lesions. Quintessence Int. 2002; 33:542–8.50. Yli-Urpo H, Lassila LV, Narhi T, Vallittu PK. Compressive strength and surface characterization of glass ionomer cements modified by particles of bioactive glass. Dent Mater. 2005; 21:201–9.
Article51. Yli-Urpo H, Narhi M, Narhi T. Compound changes and tooth mineralization effects of glass ionomer cements containing bioactive glass (S53P4), an in vivo study. Biomaterials. 2005; 26:5934–41.
Article52. Xie D, Zhao J, Weng Y, Park JG, Jiang H, Platt JA. Bioactive glass-ionomer cement with potential therapeutic function to dentin capping mineralization. Eur J Oral Sci. 2008; 116:479–87.
Article53. Ana ID, Matsuya S, Ohta M, Ishikawa K. Effects of added bioactive glass on the setting and mechanical properties of resin-modified glass ionomer cement. Biomaterials. 2003; 24:3061–7.
Article54. Hench LL. The story of Bioglass. J Mater Sci Mater Med. 2006; 17:967–78.
Article55. Mousavinasab SM, Khoroushi M, Keshani F, Hashemi S. Flexural strength and morphological characteristics of resin-modified glass-ionomer containing bioactive glass. J Contemp Dent Pract. 2011; 12:41–6.
Article56. Khoroushi M, Mousavinasab SM, Keshani F, Hashemi S. Effect of resin-modified glass ionomer containing bioactive glass on the flexural strength and morphology of demineralized dentin. Oper Dent. 2013; 38:E1–10.
Article57. Kim DA, Lee JH, Jun SK, Kim HW, Eltohamy M, Lee HH. Sol-gel-derived bioactive glass nanoparticle-incorporated glass ionomer cement with or without chitosan for enhanced mechanical and biomineralization properties. Dent Mater. 2017; 33:805–17.
Article58. Lee JH, Kang MS, Mahapatra C, Kim HW. Effect of aminated mesoporous bioactive glass nanoparticles on the differentiation of dental pulp stem cells. PLoS One. 2016; 11:e0150727.
Article59. Lee JH, El-Fiqi A, Jo JK, Kim DA, Kim SC, Jun SK, et al. Development of long-term antimicrobial poly(methyl methacrylate) by incorporating mesoporous silica nanocarriers. Dent Mater. 2016; 32:1564–74.
Article60. Padovani GC, Feitosa VP, Sauro S, Tay FR, Duran G, Paula AJ, et al. Advances in dental materials through nanotechnology: facts, perspectives and toxicological aspects. Trends Biotechnol. 2015; 33:621–36.
Article61. Oliveira-Ogliari A, Collares FM, Feitosa VP, Sauro S, Ogliari FA, Moraes RR. Methacrylate bonding to zirconia by in situ silica nanoparticle surface deposition. Dent Mater. 2015; 31:68–76.
Article62. Mabrouk M, Selim MM, Beherei H, El-Gohary MI. Effect of incorporation of nano bioactive silica into commercial glassionomer cement (GIC). J Genet Eng Biotechnol. 2012; 10:113–9.63. Choi JY, Lee HH, Kim HW. Bioactive sol-gel glass added ionomer cement for the regeneration of tooth structure. J Mater Sci Mater Med. 2008; 19:3287–94.
Article64. Saravana KR, Vijayalakshmi R. Nanotechnology in dentistry. Indian J Dent Res. 2006; 17:62–5.
Article65. Lee JH, Seo SJ, Kim HW. Bioactive glass-based nanocomposites for personalized dental tissue regeneration. Dent Mater J. 2016; 35:710–20.
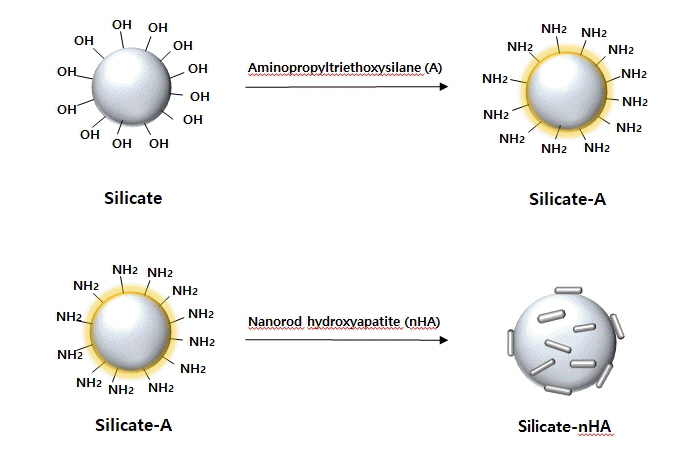
Article66. Park SJ, Gupta KC, Kim H, Kim S, Kang IK. Osteoblast behaviours on nanorod hydroxyapatite-grafted glass surfaces. Biomater Res. 2019; 23:28.
Article67. Moshaverinia A, Ansari S, Moshaverinia M, Roohpour N, Darr JA, Rehman I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomater. 2008; 4:432–40.
Article68. Arita K, Yamamoto A, Shinonaga Y, Harada K, Abe Y, Nakagawa K, et al. Hydroxyapatite particle characteristics influence the enhancement of the mechanical and chemical properties of conventional restorative glass ionomer cement. Dent Mater J. 2011; 30:672–83.69. Mitra SB, Wu D, Holmes BN. An application of nanotechnology in advanced dental materials. J Am Dent Assoc. 2003; 134:1382–90.
Article70. Saunders SA. Current practicality of nanotechnology in dentistry. Part 1: Focus on nanocomposite restoratives and biomimetics. Clin Cosmet Investig Dent. 2009; 1:47–61.
Article71. Dorozhkin SV. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 2010; 6:715–34.
Article72. Ramesh N, Moratti SC, Dias GJ. Hydroxyapatite-polymer biocomposites for bone regeneration: a review of current trends. J Biomed Mater Res B Appl Biomater. 2018; 106:2046–57.
Article73. Hannig M, Hannig C. Nanomaterials in preventive dentistry. Nat Nanotechnol. 2010; 5:565–9.
Article74. Najeeb S, Khurshid Z, Matinlinna JP, Siddiqui F, Nassani MZ, Baroudi K. Nanomodified peek dental implants: bioactive composites and surface modification-a review. Int J Dent. 2015; 2015:381759.
Article75. Khurshid Z, Zafar M, Qasim S, Shahab S, Naseem M, AbuReqaiba A. Advances in nanotechnology for restorative dentistry. Materials (Basel). 2015; 8:717–31.
Article76. Le Guehennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007; 23:844–54.
Article77. Hannig M, Hannig C. Nanotechnology and its role in caries therapy. Adv Dent Res. 2012; 24:53–7.
Article78. Curtis AR, Palin WM, Fleming GJ, Shortall AC, Marquis PM. The mechanical properties of nanofilled resin-based composites: the impact of dry and wet cyclic pre-loading on bi-axial flexure strength. Dent Mater. 2009; 25:188–97.
Article79. Terry DA. Direct applications of a nanocomposite resin system: part 1-the evolution of contemporary composite materials. Pract Proced Aesthet Dent. 2004; 16:417–22.80. Chen MH. Update on dental nanocomposites. J Dent Res. 2010; 89:549–60.
Article81. Moshaverinia A, Roohpour N, Chee WWL, Schricker SR. A review of powder modifications in conventional glass-ionomer dental cements. J Mater Chem. 2011; 21:1319–28.
Article82. IK Kang SJ Park SH Kang . SH Kang . Glass based filler for dental restoration, method for manufacturing thereof, and dental restoration comprising thereof. Korea KR patent, 10-2020-0021006. 2020 Feb 20.83. Haider A, Gupta KC, Kang IK. Morphological effects of HA on the cell compatibility of electrospun HA/PLGA composite nanofiber scaffolds. Biomed Res Int. 2014; 2014:308306.
Article84. Xia Y, Zhang F, Xie H, Gu N. Nanoparticle-reinforced resin-based dental composites. J Dent. 2008; 36:450–5.
Article85. De Caluwe T, Vercruysse CW, Fraeyman S, Verbeeck RM. The influence of particle size and fluorine content of aluminosilicate glass on the glass ionomer cement properties. Dent Mater. 2014; 30:1029–38.
Article86. Ong JL, Chan DCN. A review of hydroxapatite and its use as a coating in dental implants. Crit Rev Biomed Eng. 2017; 45:411–51.
Article87. Gu YW, Yap AUJ, Cheang P, Khor KA. Zirconia-glass ionomer cement–A potential substitute for miracle mix. Scr Mater. 2005; 52:113–6.
Article88. Huang SB, Gao SS, Yu HY. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed Mater. 2009; 4:034104.89. Huang S, Gao S, Cheng L, Yu H. Remineralization potential of nano-hydroxyapatite on initial enamel lesions: an in vitro study. Caries Res. 2011; 45:460–8.
Article90. Zakir M, Al Kheraif AA, Asif M, Wong FS, Rehman IU. A comparison of the mechanical properties of a modified silorane based dental composite with those of commercially available composite material. Dent Mater. 2013; 29:e53–9.
Article91. Yap AU, Pek YS, Kumar RA, Cheang P, Khor KA. Experimental studies on a new bioactive material: HAIonomer cements. Biomaterials. 2002; 23:955–62.
Article92. Moshaverinia A, Ansari S, Movasaghi Z, Billington RW, Darr JA, Rehman IU. Modification of conventional glass-ionomer cements with N-vinylpyrrolidone containing polyacids, nano-hydroxy and fluoroapatite to improve mechanical properties. Dent Mater. 2008; 24:1381–90.
Article93. Lee JJ, Lee YK, Choi BJ, Lee JH, Choi HJ, Son HK, et al. Physical properties of resin-reinforced glass ionomer cement modified with micro and nano-hydroxyapatite. J Nanosci Nanotechnol. 2010; 10:5270–6.
Article94. Cehreli SB, Tirali RE, Yalcinkaya Z, Cehreli ZC. Microleakage of newly developed glass carbomer cement in primary teeth. Eur J Dent. 2013; 7:15–21.95. Zainuddin N, Karpukhina N, Law RV, Hill RG. Characterisation of a remineralising Glass Carbomer® ionomer cement by MAS-NMR spectroscopy. Dent Mater. 2012; 28:1051–8.
Article96. Hasan AMHR, Sidhu SK, Nicholson JW. Fluoride release and uptake in enhanced bioactivity glass ionomer cement (“glass carbomer™”) compared with conventional and resin-modified glass ionomer cements. J Appl Oral Sci. 2019; 27:e20180230.
Article97. W Van-Duinen RN Van-Duinen . STICHTING GLASS FOR HEALTH . Self hardening glass carbomer composition. United States patent US 20060217455 A1. 2006. Sep. 28.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A comparative study on shear bond strengths influenced by time elapsed after bracket bonding with a light-cured glass ionomer cement
- In vitro study on the fluoride release from glass ionomer cements and a fluoride-containing resin
- Comparative studies on the retentive values of various dental cements used to retain orthodontic bands
- The shear bond strength of two adhesives bonded to composite resin and glass ionomer cement restorations
- The effects of surface treatments on shear bond strengths of light-cured and chemically cured glass ionomer cements to enamel