J Pathol Transl Med.
2020 Sep;54(5):367-377. 10.4132/jptm.2020.07.21.
Pathologic interpretation of endoscopic ultrasound–guided fine needle aspiration cytology/biopsy for pancreatic lesions
- Affiliations
-
- 1Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2506270
- DOI: http://doi.org/10.4132/jptm.2020.07.21
Abstract
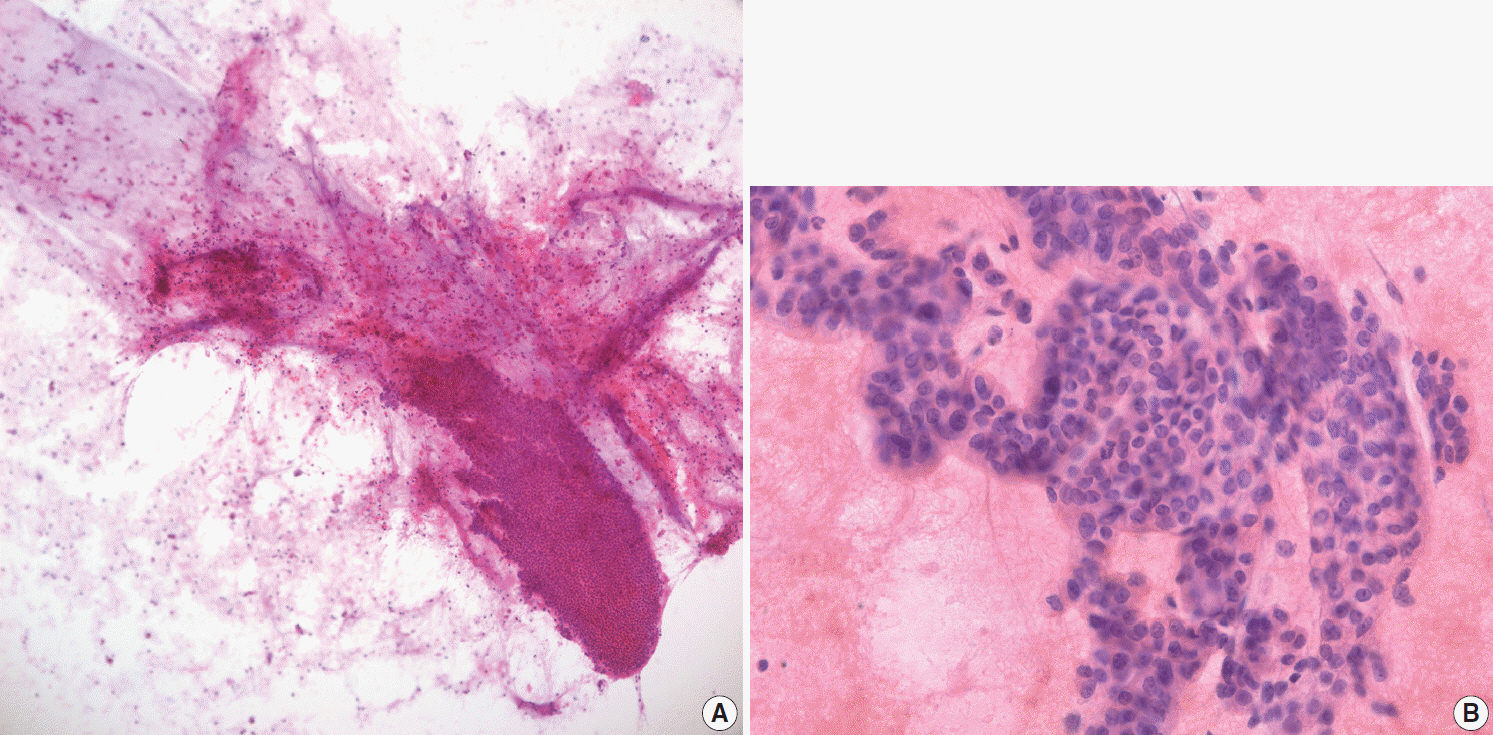
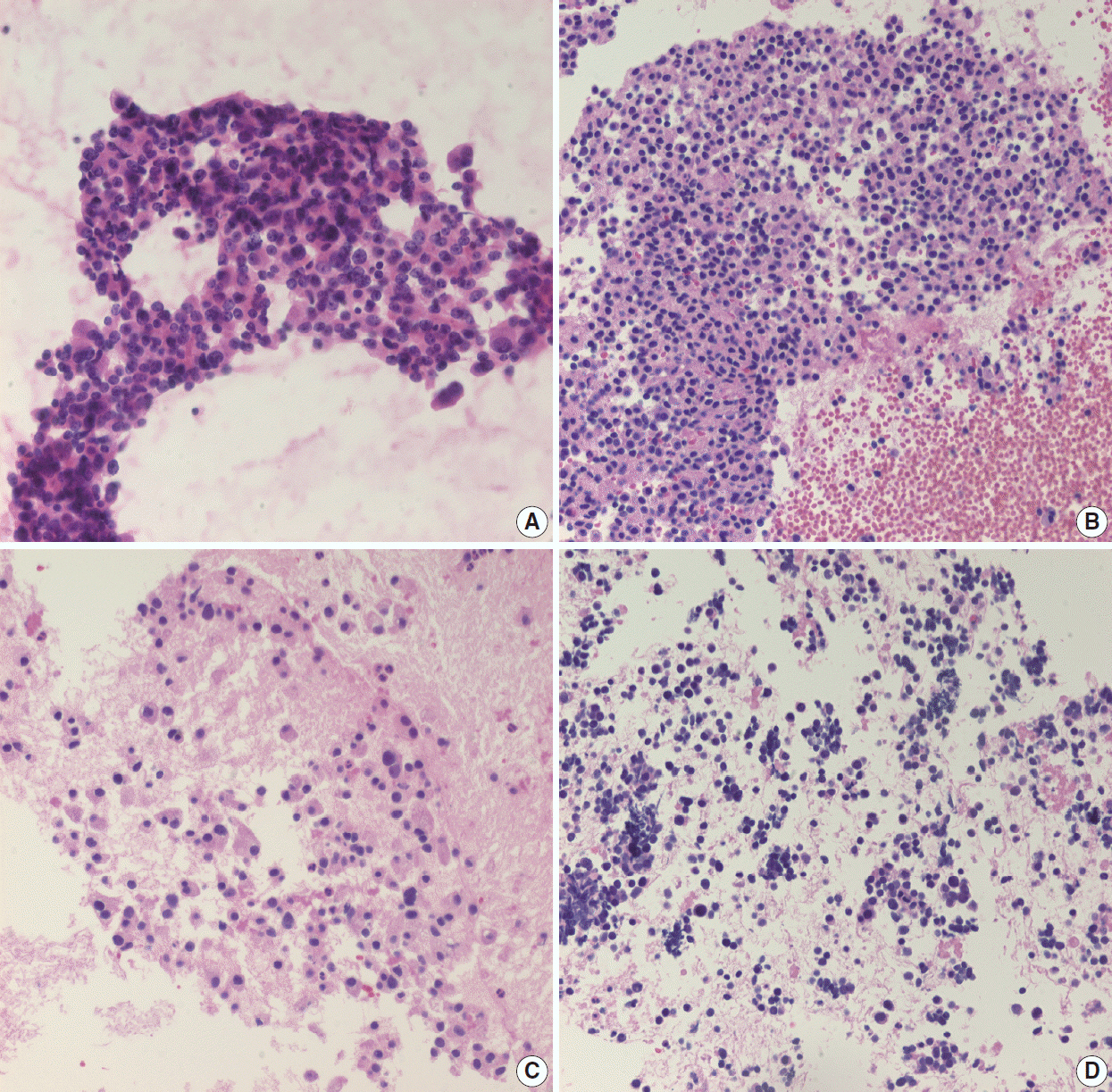
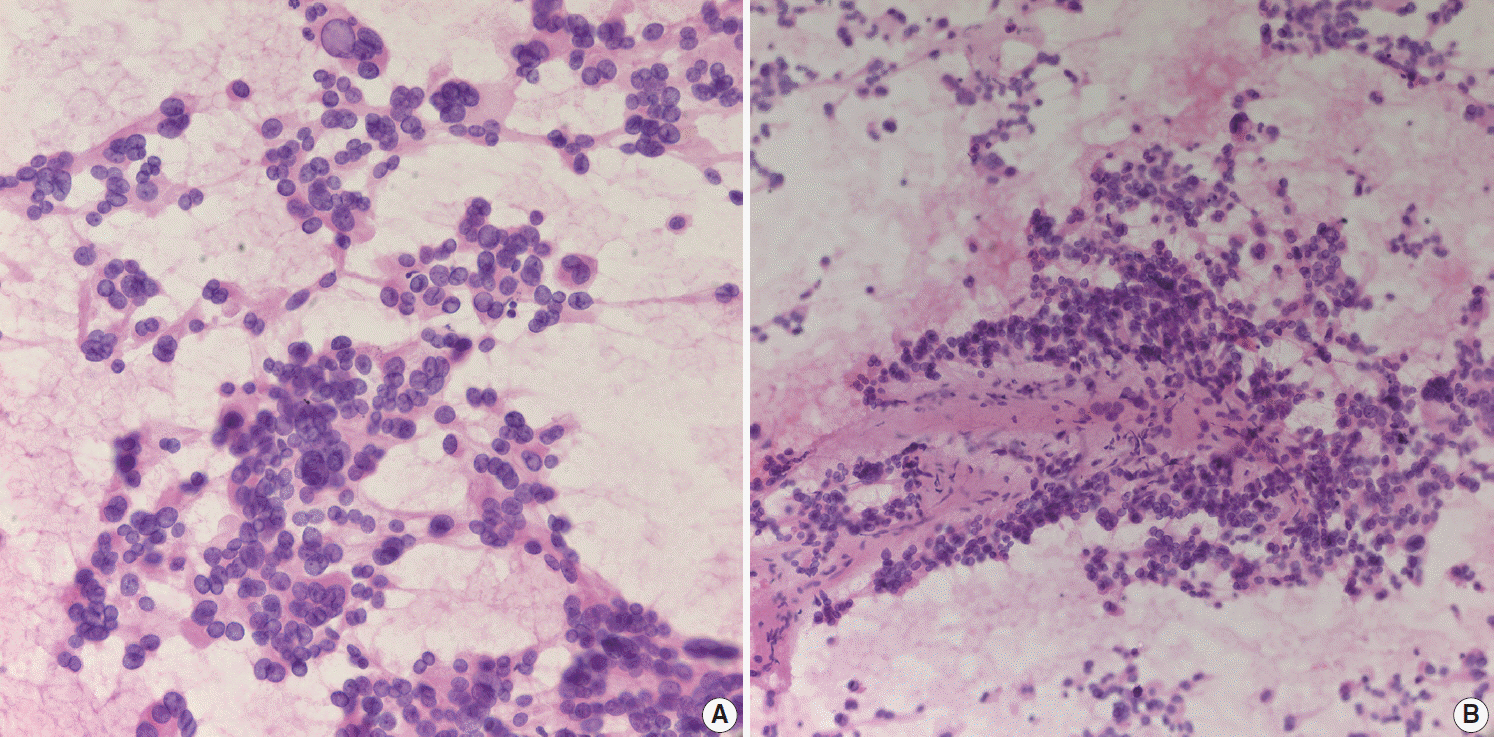
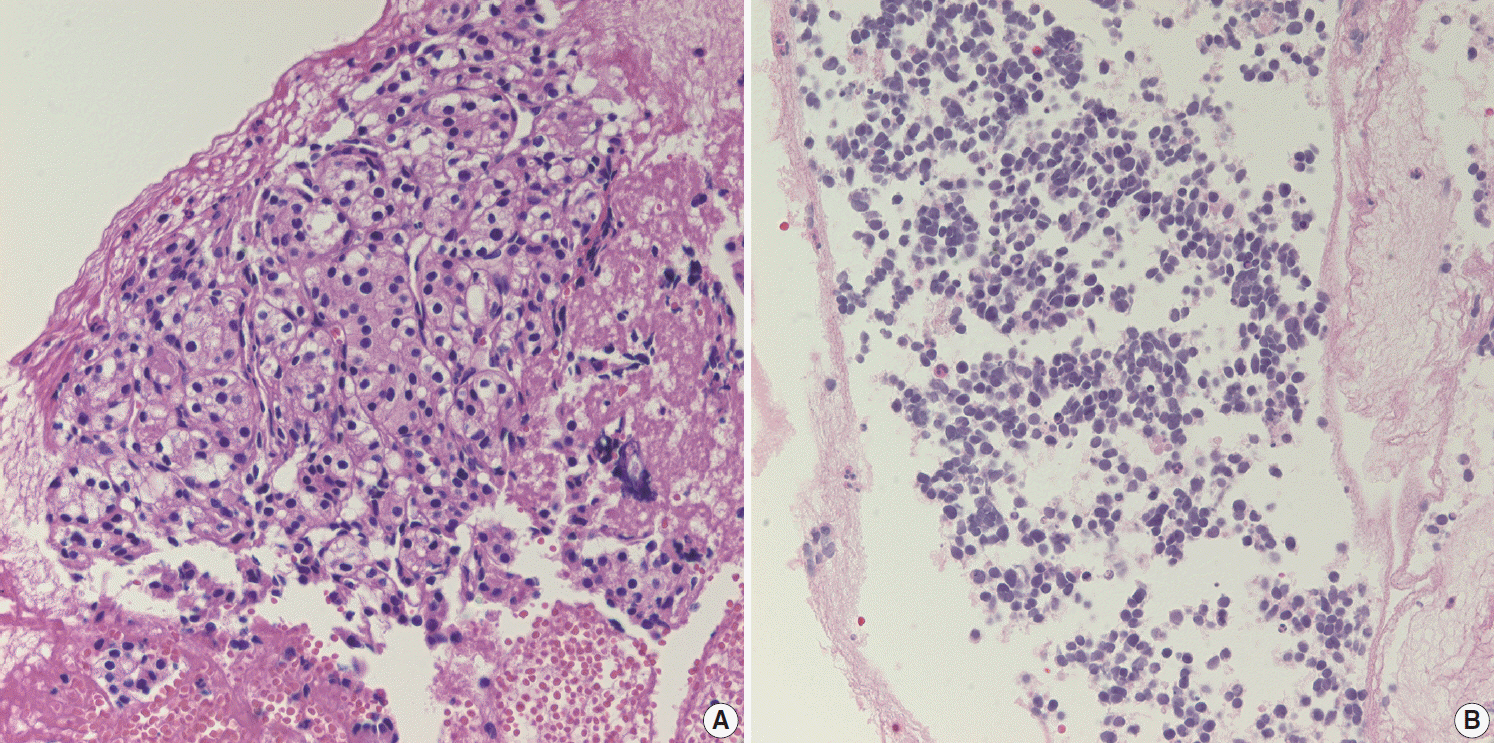
- Pathologic interpretation of endoscopic ultrasound–guided fine needle aspiration (EUS-FNA) cytology/biopsy specimens is one of the most challenging tasks in cytology and surgical pathology practice, as the procedure often yields minimal amounts of diagnostic material and contains contaminants, such as blood cells and normal intestinal mucosa. EUS-FNA cytology/biopsy will nevertheless become a more popular procedure for evaluation of various pancreatic lesions because they are difficult to approach with conventional endoscopic procedures. Pathologists should understand the structural differences and limitations of EUS-FNA that make pathologic diagnosis difficult. Ancillary tests are available for differential diagnosis of EUS-FNA for various pancreatic lesions. Immunostains are the most commonly used ancillary tests, and pathologists should able to choose the necessary panel for differential diagnosis. Pathologists should review clinical history and radiologic and/or EUS findings before selecting an immunostain panel and making a pathologic diagnosis. In addition, one’s threshold of malignancy should be adjusted according to the appropriate clinical setting to avoid under-evaluation of pathologic diagnoses. Clinico-pathologic correlation is essential in pathologic evaluation of EUS-FNA for pancreatic lesions. Pathologists can reduce errors by correlating clinical and radiologic findings when evaluating EUS-FNA. Some molecular tests can be applied in differential diagnosis of pancreatic neoplastic and cystic lesions. Molecular data should be used as supportive evidence of a specific disease entity, rather than direct evidence, and should be correlated with clinico-pathologic findings to avoid errors in pathologic diagnosis.
Figure
Reference
-
References
1. DiMagno EP, Buxton JL, Regan PT, et al. Ultrasonic endoscope. Lancet. 1980; 1:629–31.
Article2. Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992; 38:172–3.
Article3. Chun JW, Lee K, Lee SH, et al. Comparison of liquid-based cytology with conventional smear cytology for EUS-guided FNA of solid pancreatic masses: a prospective randomized noninferiority study. Gastrointest Endosc. 2020; 91:837–46.
Article4. Mitoro A, Nishikawa T, Yoshida M, et al. Diagnostic efficacy of liquid-based cytology in endoscopic ultrasound-guided fine needle aspiration for pancreatic mass lesions during the learning curve: a retrospective study. Pancreas. 2019; 48:686–9.5. Yung RC, Otell S, Illei P, et al. Improvement of cellularity on cell block preparations using the so-called tissue coagulum clot method during endobronchial ultrasound-guided transbronchial fine-needle aspiration. Cancer Cytopathol. 2012; 120:185–95.
Article6. Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010; 105:2079–84.
Article7. Wittmann J, Kocjan G, Sgouros SN, Deheragoda M, Pereira SP. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and trucut needle biopsy: a prospective study. Cytopathology. 2006; 17:27–33.
Article8. Lee YN, Moon JH, Choi HJ, et al. Tissue acquisition for diagnosis of biliary strictures using peroral cholangioscopy or endoscopic ultrasound-guided fine-needle aspiration. Endoscopy. 2019; 51:50–9.
Article9. Lee JK, Lee KT, Choi ER, et al. A prospective, randomized trial comparing 25-gauge and 22-gauge needles for endoscopic ultrasoundguided fine needle aspiration of pancreatic masses. Scand J Gastroenterol. 2013; 48:752–7.
Article10. Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018; 31:1770–86.
Article11. Misdraji J, Centeno BA, Pitman MB. Ancillary tests in the diagnosis of liver and pancreatic neoplasms. Cancer Cytopathol. 2018; 126 Suppl 8:672–90.
Article12. Li J, Lin JP, Shi LH, et al. How reliable is the Ki-67 cytological index in grading pancreatic neuroendocrine tumors? A meta-analysis. J Dig Dis. 2016; 17:95–103.
Article13. Weynand B, Borbath I, Bernard V, et al. Pancreatic neuroendocrine tumour grading on endoscopic ultrasound-guided fine needle aspiration: high reproducibility and inter-observer agreement of the Ki-67 labelling index. Cytopathology. 2014; 25:389–95.
Article14. Hwang HS, Kim Y, An S, et al. Grading by the Ki-67 labeling index of endoscopic ultrasound-guided fine needle aspiration biopsy specimens of pancreatic neuroendocrine tumors can be underestimated. Pancreas. 2018; 47:1296–303.
Article15. Kim SA, Kim MS, Kim MS, et al. Pleomorphic solid pseudopapillary neoplasm of the pancreas: degenerative change rather than high-grade malignant potential. Hum Pathol. 2014; 45:166–74.
Article16. Kim S, Bae H, Choi M, et al. Isolated mass-forming IgG4-related cholangitis as an initial clinical presentation of systemic IgG4-related disease. J Pathol Transl Med. 2016; 50:300–5.
Article17. Lee HE, Zhang L. Immunoglobulin G4-related hepatobiliary disease. Semin Diagn Pathol. 2019; 36:423–33.
Article18. Heymann JJ, Siddiqui MT. Ancillary techniques in cytologic specimens obtained from solid lesions of the pancreas: a review. Acta Cytol. 2020; 64:103–23.
Article19. Layfield LJ, Ehya H, Filie AC, et al. Utilization of ancillary studies in the cytologic diagnosis of biliary and pancreatic lesions: the Papanicolaou Society of Cytopathology guidelines for pancreatobiliary cytology. Diagn Cytopathol. 2014; 42:351–62.
Article20. Soyer OM, Baran B, Ormeci AC, et al. Role of biochemistry and cytological analysis of cyst fluid for the differential diagnosis of pancreatic cysts: a retrospective cohort study. Medicine (Baltimore). 2017; 96:e5513.21. Kubiliun N, Ribeiro A, Fan YS, et al. EUS-FNA with rescue fluorescence in situ hybridization for the diagnosis of pancreatic carcinoma in patients with inconclusive on-site cytopathology results. Gastrointest Endosc. 2011; 74:541–7.
Article22. Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011; 3:92ra66.
Article23. Jones M, Zheng Z, Wang J, et al. Impact of next-generation sequencing on the clinical diagnosis of pancreatic cysts. Gastrointest Endosc. 2016; 83:140–8.
Article24. Ngamruengphong S, Lennon AM. Analysis of pancreatic cyst fluid. Surg Pathol Clin. 2016; 9:677–84.
Article25. Rosenbaum MW, Jones M, Dudley JC, Le LP, Iafrate AJ, Pitman MB. Next-generation sequencing adds value to the preoperative diagnosis of pancreatic cysts. Cancer Cytopathol. 2017; 125:41–7.
Article26. Hartley CP, Mahajan AM, Selvaggi SM, Rehrauer WM. FNA smears of pancreatic ductal adenocarcinoma are superior to formalin-fixed paraffin-embedded tissue as a source of DNA: Comparison of targeted KRAS amplification and genotyping in matched preresection and postresection samples. Cancer Cytopathol. 2017; 125:838–47.
Article27. Park YJ, Kim GH, Park DY, et al. Histopathologic discrepancies between endoscopic forceps biopsy and endoscopic resection specimens in superficial esophageal squamous neoplasms. J Gastroenterol Hepatol. 2019; 34:1058–65.
Article28. Ilie M, Long-Mira E, Bence C, et al. Comparative study of the PDL1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol. 2016; 27:147–53.
Article29. Cros J, Raffenne J, Couvelard A, Pote N. Tumor heterogeneity in pancreatic adenocarcinoma. Pathobiology. 2018; 85:64–71.
Article30. Prasetyanti PR, Medema JP. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer. 2017; 16:41.
Article31. Allenson K, Castillo J, San Lucas FA, et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann Oncol. 2017; 28:741–7.32. Takai E, Totoki Y, Nakamura H, Kato M, Shibata T, Yachida S. Clinical utility of circulating tumor DNA for molecular assessment and precision medicine in pancreatic cancer. Adv Exp Med Biol. 2016; 924:13–7.
Article33. Torres C, Grippo PJ. Pancreatic cancer subtypes: a roadmap for precision medicine. Ann Med. 2018; 50:277–87.
Article34. Chantrill LA, Nagrial AM, Watson C, et al. Precision medicine for advanced pancreas cancer: the Individualized Molecular Pancreatic Cancer Therapy (IMPaCT) trial. Clin Cancer Res. 2015; 21:2029–37.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Repeated Endoscopic Ultrasound-Guided Fine Needle Aspiration for Inconclusive Initial Cytology Result
- Endoscopic Ultrasound-Guided Fine Needle Aspiration in Cystic Pancreatic Lesions
- The Usefulness of Ultrasound-Guided Fine Needle Aspiration in Breast Lesions
- Endoscopic Ultrasound-Fine Needle Aspiration versus Core Biopsy for the Diagnosis of Subepithelial Tumors
- Fine-Needle Biopsy: Should This Be the First Choice in Endoscopic Ultrasound-Guided Tissue Acquisition?