J Korean Med Sci.
2020 Sep;35(35):e284. 10.3346/jkms.2020.35.e284.
Impact of Molecular Drug Susceptibility Testing on the Time to Multidrug-resistant Tuberculosis Treatment Initiation
- Affiliations
-
- 1Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea
- 2Department of Chest Medicine, Masan National Tuberculosis Hospital, Masan, Korea
- 3Department of Internal Medicine, Chonnam National University Hospital, Gwangju, Korea
- 4Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 5Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2506083
- DOI: http://doi.org/10.3346/jkms.2020.35.e284
Abstract
- Background
The purpose of this study was to evaluate the current status and trends in the coverage of molecular drug susceptibility testing (mDST), and the impact of mDST on the time to multidrug-resistant tuberculosis (MDR-TB) treatment initiation in Korea.
Methods
We included confirmed rifampin-resistant (RR)/MDR-TB patients who submitted application forms for novel drug uses to the National TB Expert Review Committee from September 1, 2016 to November 30, 2019. We retrospectively reviewed their medical records.
Results
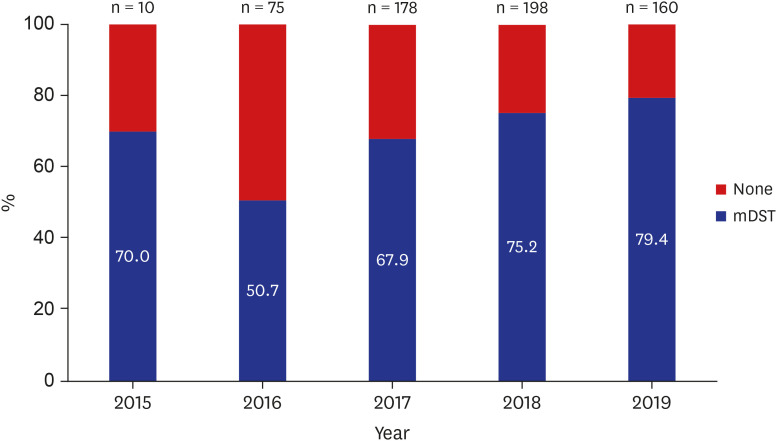
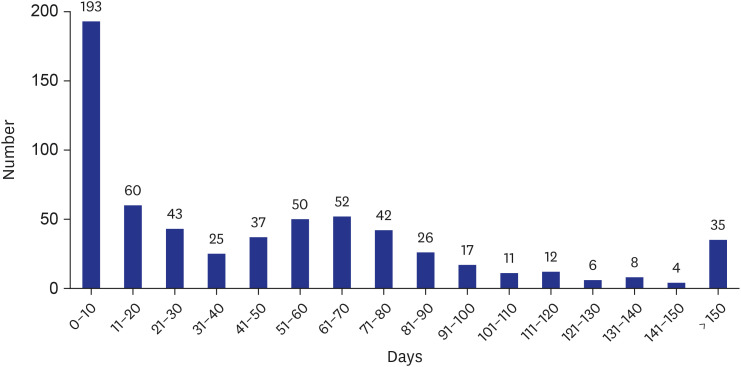
Of the 621 MDR/RR-TB patients, mDST was performed in 442 (71.2%); Xpert MTB/ RIF (Xpert) alone in 109 (17.6%), MTBDRplus line probe assay (LPA) alone in 199 (32.0%), and both Xpert and LPA in 134 (21.6%) patients. The coverage rate of mDST has gradually increased to 70% in 2015, 50.7% in 2016, 67.9% in 2017, 75.2% in 2018, and 79.4% in 2019 (p for trend < 0.001). Median time to MDR-TB treatment initiation was 35 days (interquartile range 25–75 0–72), which has gradually decreased during the study period (p< 0.001).Independent predictors of shorter time to MDR-TB treatment initiation were retreatment case (adjusted hazard ratio [aHR], 1.30; 95% confidence interval [CI], 1.10–1.54), Xpert testing (aHR, 2.42; 95% CI, 2.03–2.88), and LPA testing (aHR, 1.83; 95% CI, 1.55–2.16).Transfer to another healthcare facility was inversely related to shorter time to treatment initiation (aHR, 0.74; 95% CI, 0.63–0.88).
Conclusion
mDST coverage is gradually increasing and contributes to reducing the time to MDR-TB treatment initiation. Further efforts are needed to achieve universal access to mDST and to properly integrate mDST into routine clinical practice.
Figure
Reference
-
1. Nathavitharana RR, Lederer P, Tierney DB, Nardell E. Treatment as prevention and other interventions to reduce transmission of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2019; 23(4):396–404. PMID: 31064617.
Article2. Lange C, Dheda K, Chesov D, Mandalakas AM, Udwadia Z, Horsburgh CR Jr. Management of drug-resistant tuberculosis. Lancet. 2019; 394(10202):953–966. PMID: 31526739.
Article3. Cirillo DM, Miotto P, Tortoli E. Evolution of phenotypic and molecular drug susceptibility testing. Adv Exp Med Biol. 2017; 1019:221–246. PMID: 29116638.
Article4. World Health Organization. Policy statement. WHO policy statement: molecular line probe assays for rapid screening of patients at risk of multidrug-resistant tuberculosis. Updated 2008. Accessed May 30, 2020. https://www.who.int/tb/features_archive/policy_statement.pdf.5. World Health Organization. Policy statement. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. Updated 2011. Accessed May 30, 2020. https://apps.who.int/iris/bitstream/handle/10665/44586/9789241501545_eng.pdf?sequence=1.6. Jacobson KR, Theron D, Kendall EA, Franke MF, Barnard M, van Helden PD, et al. Implementation of genotype MTBDRplus reduces time to multidrug-resistant tuberculosis therapy initiation in South Africa. Clin Infect Dis. 2013; 56(4):503–508. PMID: 23090928.
Article7. Kipiani M, Mirtskhulava V, Tukvadze N, Magee M, Blumberg HM, Kempker RR. Significant clinical impact of a rapid molecular diagnostic test (genotype MTBDRplus assay) to detect multidrug-resistant tuberculosis. Clin Infect Dis. 2014; 59(11):1559–1566. PMID: 25091301.
Article8. Eliseev P, Balantcev G, Nikishova E, Gaida A, Bogdanova E, Enarson D, et al. The impact of a line probe assay based diagnostic algorithm on time to treatment initiation and treatment outcomes for multidrug resistant TB patients in Arkhangelsk region, Russia. PLoS One. 2016; 11(4):e0152761. PMID: 27055269.
Article9. Stagg HR, White PJ, Riekstiņa V, Cīrule A, Šķenders Ģ, Leimane V, et al. Decreased time to treatment initiation for multidrug-resistant tuberculosis patients after use of Xpert MTB/RIF test, Latvia. Emerg Infect Dis. 2016; 22(3):482–490. PMID: 26889608.
Article10. Boyd R, Ford N, Padgen P, Cox H. Time to treatment for rifampicin-resistant tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2017; 21(11):1173–1180. PMID: 29037299.
Article11. Cox H, Dickson-Hall L, Ndjeka N, Van't Hoog A, Grant A, Cobelens F, et al. Delays and loss to follow-up before treatment of drug-resistant tuberculosis following implementation of Xpert MTB/RIF in South Africa: a retrospective cohort study. PLoS Med. 2017; 14(2):e1002238. PMID: 28222095.
Article12. Iruedo J, O'Mahony D, Mabunda S, Wright G, Cawe B. The effect of the Xpert MTB/RIF test on the time to MDR-TB treatment initiation in a rural setting: a cohort study in South Africa's Eastern Cape Province. BMC Infect Dis. 2017; 17(1):91. PMID: 28109255.
Article13. Chen Y, Yuan Z, Shen X, Wu J, Wu Z, Xu B. Time to multidrug-resistant tuberculosis treatment initiation in association with treatment outcomes in Shanghai, China. Antimicrob Agents Chemother. 2018; 62(4):e02259–17. PMID: 29437632.
Article14. Htun YM, Khaing TM, Yin Y, Myint Z, Aung ST, Hlaing TM, et al. Delay in diagnosis and treatment among adult multidrug resistant tuberculosis patients in Yangon Regional Tuberculosis Center, Myanmar: a cross-sectional study. BMC Health Serv Res. 2018; 18(1):878. PMID: 30458776.
Article15. Obregón G, Zevallos K, Alarcón V, Puyén ZM, Chávez Inagaki O, Mendoza-Ticona A, et al. Rapid drug susceptibility testing and treatment outcomes for multidrug-resistant tuberculosis in Peru. Int J Tuberc Lung Dis. 2018; 22(11):1350–1357. PMID: 30355416.
Article16. Jo EJ, Park S, Lee KM, Kim I, Eom JS, Kim MH, et al. Time to appropriate treatment in patients with multidrug-resistant tuberculosis in South Korea: Are we still in 2010? PLoS One. 2019; 14(4):e0216084. PMID: 31022260.
Article17. World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Updated 2019. Accessed May 30, 2020. https://apps.who.int/iris/bitstream/handle/10665/311389/9789241550529-eng.pdf?ua=1.18. World Health Organization. Global tuberculosis report 2019. Updated 2019. Accessed May 30, 2020. https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1.19. Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korean Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 3rd ed. Updated 2017. Accessed May 30, 2020. https://www.lungkorea.org/bbs/index.html?code=guide&page=2.20. Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korean Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 4th ed. Updated 2020. Accessed May 30, 2020. https://www.lungkorea.org/bbs/index.html?code=guide&page=1.21. Joh JS, Lee CH, Lee JE, Park YK, Bai GH, Kim EC, et al. The interval between initiation of anti-tuberculosis treatment in patients with culture-positive pulmonary tuberculosis and receipt of drug-susceptibility test results. J Korean Med Sci. 2007; 22(1):26–29. PMID: 17297247.
Article22. Korea Centers for Disease Control & Prevention. Annual report on the notified tuberculosis patients in Korea 2019. Updated 2020. Accessed May 30, 2020. http://tbzero.cdc.go.kr/tbzero/board/boardView.do.23. Lisboa M, Fronteira I, Colove E, Nhamonga M, Martins MD. Time delay and associated mortality from negative smear to positive Xpert MTB/RIF test among TB/HIV patients: a retrospective study. BMC Infect Dis. 2019; 19(1):18. PMID: 30616533.
Article24. Mann G, Squire SB, Bissell K, Eliseev P, Du Toit E, Hesseling A, et al. Beyond accuracy: creating a comprehensive evidence base for TB diagnostic tools. Int J Tuberc Lung Dis. 2010; 14(12):1518–1524. PMID: 21144235.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medical Management of Drug-Resistant Tuberculosis
- Drug Resistance Patterns of Multidrug- and Extensively Drug-Resistant Tuberculosis in Korea: Amplification of Resistance to Oral Second-line Drugs
- Pediatric tuberculosis and drug resistance
- Rifampin-resistant Relapsed Tuberculosis Confirmed by Molecular Technique
- Pulmonary Tuberculosis Diagnosis: Where We Are?