Arch Hand Microsurg.
2020 Sep;25(3):225-231. 10.12790/ahm.20.0036.
Lymphatic Vessels Mapping in the Lower Extremities of a Healthy Asian Population
- Affiliations
-
- 1Department of Plastic and Reconstructive Surgery, Korea University Ansan Hospital, Ansan, Korea
- KMID: 2505904
- DOI: http://doi.org/10.12790/ahm.20.0036
Abstract
- Purpose
Intraoperative indocyanine green (ICG) lymphography is an effective tool to obtain real-time video images of functioning lymph vessels in edematous limbs. However, it is difficult to identify the course of lymph vessels in obese patients or patients with large dermal backflow. Without the image, surgeons have to rely on their experience when performing the skin incision to locate the lymphatic vessels. This study focused on elucidating lymphatic vessel flow patterns in healthy lower extremities in an Asian population to refer these findings for lymphedema treatment.
Methods
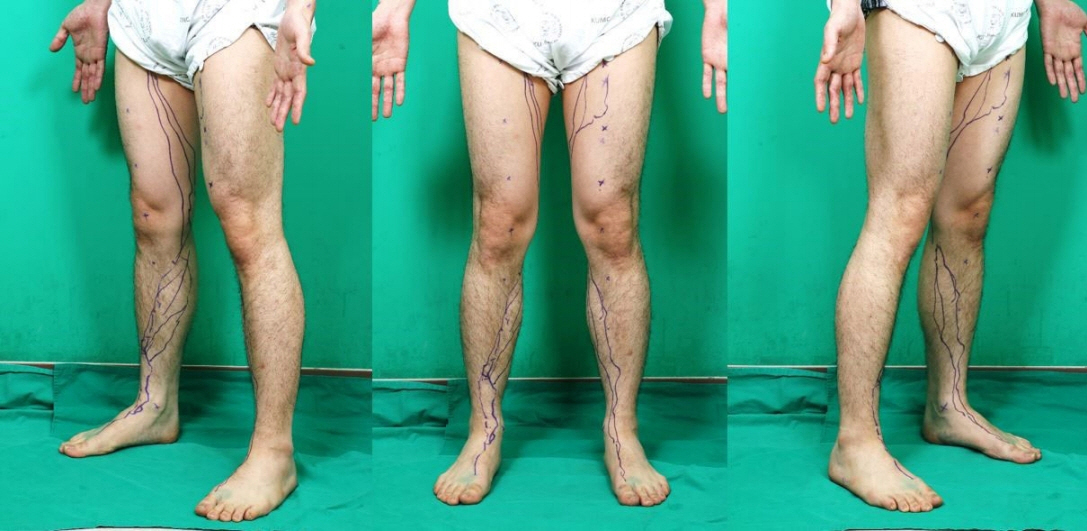
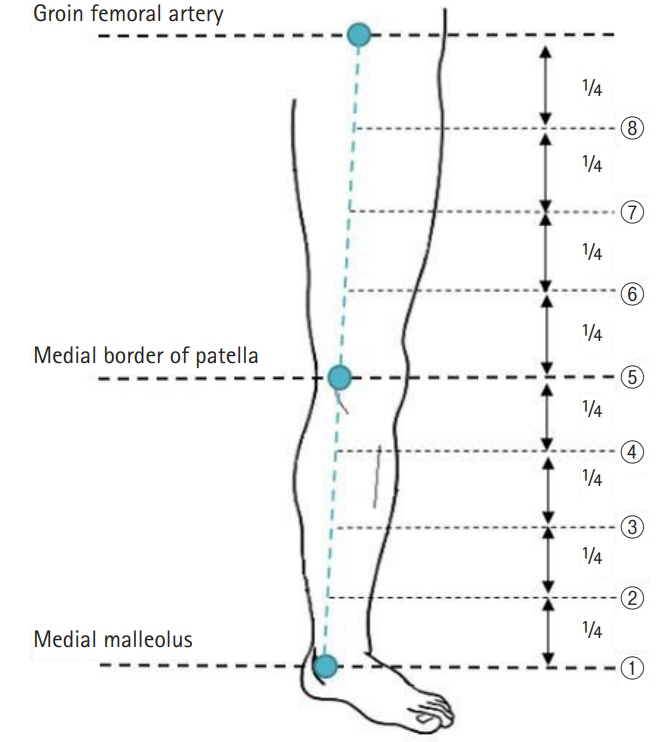
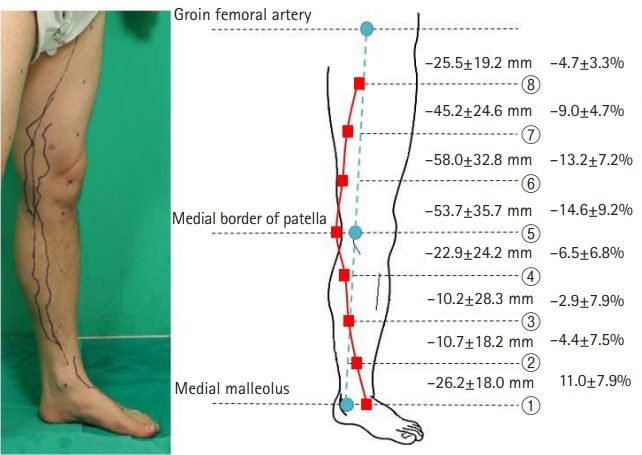
ICG fluorescence lymphography was performed by injecting 0.2 mL of ICG into the first web space of the foot. After 4 hours, fluorescence images of lymphatic vessels were obtained and the lymphatic vessels were marked. Three landmarks were designated; the medial malleolus, the medial patellar border, and the groin femoral artery. Straight lines connecting the points were drawn, and the distance between the connected lines and the marked lymphatic vessels was measured in eight points.
Results
Fifteen subjects with healthy lower extremities (15 right and 15 left) were included. The average course of the main lymph vessels passed 26.2±18.0 mm dorsally to the medial malleolus, 53.7±35.7 mm medially to the medial patellar border, and 25.5±19.2 mm medially to the three-quarters point of the upper landmark line.
Conclusion
The main functioning lymphatic vessel largely follows the great saphenous vein course, passes in front of the medial malleolus, runs over the posterior border of the medial epicondyle at the knee level, and travels anteriorly toward the inguinal lymph nodes.
Figure
Reference
-
1. Ogata F, Azuma R, Kikuchi M, Koshima I, Morimoto Y. Novel lymphography using indocyanine green dye for near-infrared fluorescence labeling. Ann Plast Surg. 2007; 58:652–5.
Article2. Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema: a comprehensive review. Ann Plast Surg. 2007; 59:464–72.3. Kinmonth JB, Taylor GW. The lymphatic circulation in lymphedema. Ann Surg. 1954; 139:129–36.
Article4. Yamamoto T, Narushima M, Kikuchi K, et al. Lambda-shaped anastomosis with intravascular stenting method for safe and effective lymphaticovenular anastomosis. Plast Reconstr Surg. 2011; 127:1987–92.
Article5. Campisi C, Boccardo F. Microsurgical techniques for lymphedema treatment: derivative lymphatic-venous microsurgery. World J Surg. 2004; 28:609–13.
Article6. Koshima I, Inagawa K, Urushibara K, Moriguchi T. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg. 2000; 16:437–42.
Article7. Koshima I, Nanba Y, Tsutsui T, Takahashi Y, Itoh S. Long-term follow-up after lymphaticovenular anastomosis for lymphedema in the leg. J Reconstr Microsurg. 2003; 19:209–15.
Article8. Yamamoto T, Narushima M, Yoshimatsu H, et al. Minimally invasive lymphatic supermicrosurgery (MILS): indocyanine green lymphography-guided simultaneous multisite lymphaticovenular anastomoses via millimeter skin incisions. Ann Plast Surg. 2014; 72:67–70.9. Yamamoto T, Koshima I, Yoshimatsu H, Narushima M, Miahara M, Iida T. Simultaneous multi-site lymphaticovenular anastomoses for primary lower extremity and genital lymphoedema complicated with severe lymphorrhea. J Plast Reconstr Aesthet Surg. 2011; 64:812–5.
Article10. Ogata F, Narushima M, Mihara M, Azuma R, Morimoto Y, Koshima I. Intraoperative lymphography using indocyanine green dye for near-infrared fluorescence labeling in lymphedema. Ann Plast Surg. 2007; 59:180–4.
Article11. Yamamoto T, Matsuda N, Doi K, et al. The earliest finding of indocyanine green lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer treatment: the modified dermal backflow stage and concept of subclinical lymphedema. Plast Reconstr Surg. 2011; 128:314e–321e.12. Yamamoto T, Narushima M, Doi K, et al. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: the generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast Reconstr Surg. 2011; 127:1979–86.
Article13. Yamamoto T, Yamamoto N, Doi K, et al. Indocyanine green-enhanced lymphography for upper extremity lymphedema: a novel severity staging system using dermal backflow patterns. Plast Reconstr Surg. 2011; 128:941–7.14. Unno N, Nishiyama M, Suzuki M, et al. Quantitative lymph imaging for assessment of lymph function using indocyanine green fluorescence lymphography. Eur J Vasc Endovasc Surg. 2008; 36:230–6.
Article15. Seki Y, Yamamoto T, Yoshimatsu H, et al. The superior-edge-of-the-knee incision method in lymphaticovenular anastomosis for lower extremity lymphedema. Plast Reconstr Surg. 2015; 136:665e–675e.
Article16. Amore M, Tapia L, Mercado D, Pattarone G, Ciucci J. Lymphedema: a general outline of its anatomical base. J Reconstr Microsurg. 2016; 32:2–9.
Article17. Shah C, Vicini FA. Breast cancer-related arm lymphedema: incidence rates, diagnostic techniques, optimal management and risk reduction strategies. Int J Radiat Oncol Biol Phys. 2011; 81:907–14.
Article18. Tomczak H, Nyka W, Lass P. Lymphoedema: lymphoscintigraphy versus other diagnostic techniques: a clinician’s point of view. Nucl Med Rev Cent East Eur. 2005; 8:37–43.19. Unno N, Inuzuka K, Suzuki M, et al. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J Vasc Surg. 2007; 45:1016–21.
Article20. Unno N, Nishiyama M, Suzuki M, et al. A novel method of measuring human lymphatic pumping using indocyanine green fluorescence lymphography. J Vasc Surg. 2010; 52:946–52.
Article21. Szuba A, Rockson SG. Lymphedema: classification, diagnosis and therapy. Vasc Med. 1998; 3:145–56.
Article22. Hope-Ross M, Yannuzzi LA, Gragoudas ES, et al. Adverse reactions due to indocyanine green. Ophthalmology. 1994; 101:529–33.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lymphatic vessel mapping in the upper extremities of a healthy Korean population
- Pedal Indirect Lymphangiography
- Risk Assessment of Dermatolymphangioadenitis by Lymphoscintigraphy in Patients with Lower Extremity Lymphedema
- Interplay between Inflammatory Responses and Lymphatic Vessels
- Efficacy and patient satisfaction regarding lymphovenous bypass with sleeve-in anastomosis for extremity lymphedema