J Liver Cancer.
2020 Mar;20(1):41-52. 10.17998/jlc.20.1.41.
Hepatocellular Carcinoma in Korea Between 2008 and 2011: an Analysis of Korean Nationwide Cancer Registry
- Affiliations
-
- 1Department of Internal Medicine, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea
- 2Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
- 3Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 4Center for Liver Cancer, Research Institute and Hospital, Goyang, Korea
- 5Department of Radiology, National Cancer Center, Goyang, Korea
- 6Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea
- 7Department of Surgery, Seoul National University College of Medicine, Seoul, Korea
- 8Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea
- 9Cancer Registration and Statistic Branch, National Cancer Control Institute, National Cancer Center, Goyang, Korea
- 10Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2505841
- DOI: http://doi.org/10.17998/jlc.20.1.41
Abstract
- Backgrounds/Aims
Backgrounds/Aims: In Korea, hepatocellular carcinoma (HCC) is the sixth most common cancer and results in the second-highest cancer death rate among all cancers. We aimed to describe the characteristics of patients who were newly diagnosed with HCC in Korea between 2008 and 2011.
Methods
The Korean Primary Liver Cancer Registry (KPLCR) is a random sample consisting of approximately 15% of patients with newly diagnosed primary liver cancer registered in the Korean Central Cancer Registry. We investigated the baseline characteristics, treatment modalities, and overall survival (OS) of patients with HCC registered in the KPLCR between 2008 and 2011.
Results
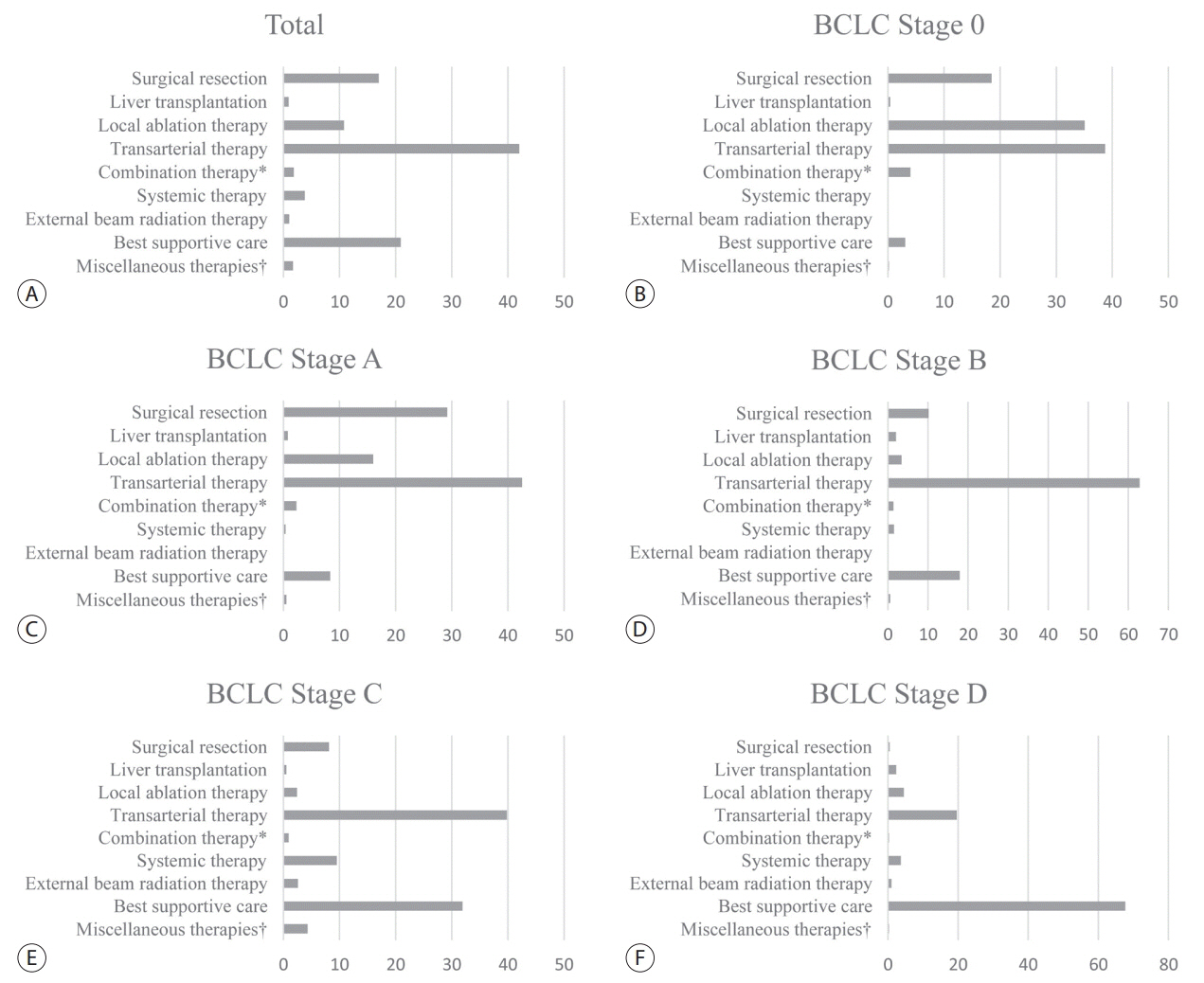
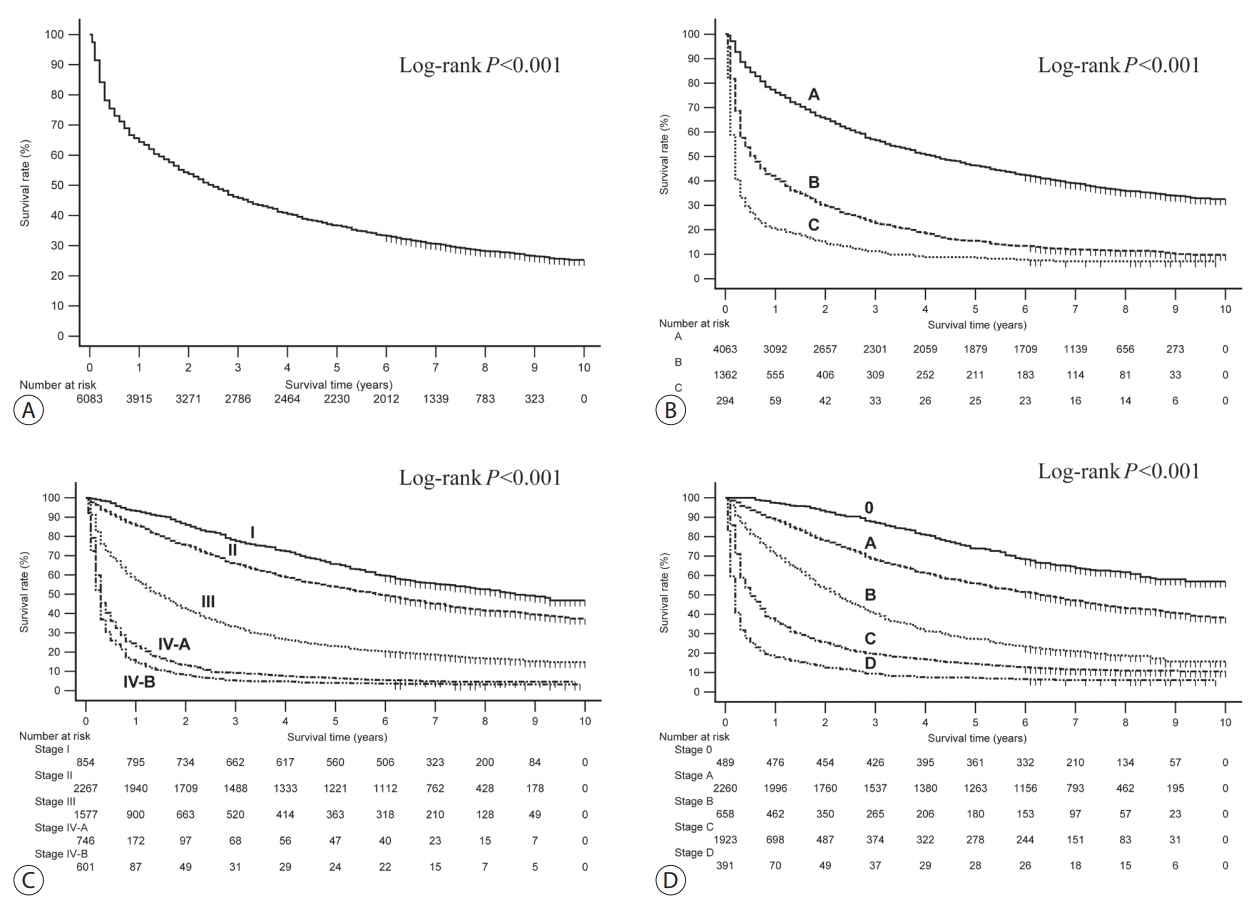
A total of 6,083 patients were histologically or radiologically diagnosed with HCC. The hepatitis B virus was the predominant HCC etiology (72.0%). According to the Barcelona Clinic Liver Cancer (BCLC) staging system, stages 0, A, B, C, and D accounted for 8.6%, 39.7%, 11.5%, 33.8%, and 6.9%, respectively. Transarterial therapy (41.7%) was the most commonly performed initial treatment, followed by best supportive care (21.7%), surgical resection (16.7%), and local ablation therapies (10.6%). The overall rate of adherence to the BCLC treatment guideline was only 37.7%. The 1-, 3-, and 5-year OS rates were 65.6%, 46.2%, and 36.8%, respectively.
Conclusions
Between 2008 and 2011, approximately half of patients with HCC (48.3%) were candidates for curative treatment (BCLC stage 0 or A), but one-third of patients (33.8%) had advanced HCC (BCLC stage C). Transarterial therapy was the most commonly conducted initial treatment and the 5-year OS rate was 36.8% in this period.
Figure
Cited by 2 articles
-
Outcome of Immune Checkpoint Inhibitor and Molecular Target Agent Combination for Advanced Hepatocellular Carcinoma: Beyond Sorafenib Era
Nae-Yun Heo
Korean J Gastroenterol. 2021;77(3):145-147. doi: 10.4166/kjg.2021.013.Advancing Korean nationwide registry for hepatocellular carcinoma: a systematic sampling approach utilizing the Korea Central Cancer Registry database
Bo Hyun Kim, E Hwa Yun, Jeong-Hoon Lee, Geun Hong, Jun Yong Park, Ju Hyun Shim, Eunyang Kim, Hyun-Joo Kong, Kyu-Won Jung, Young-Suk Lim
J Liver Cancer. 2024;24(1):57-61. doi: 10.17998/jlc.2024.03.03.
Reference
-
1. Statistics Korea. Annual report on the causes of death statistics 2017. Daejeon: Statistics Korea;2018.2. Kweon SS. Updates on cancer epidemiology in Korea, 2018. Chonnam Med J. 2018; 54:90–100.3. National Cancer Center. Korea Central Cancer Registry. Annual report of cancer statistics in Korea in 2016. Goyang: National Cancer Center;2018.4. McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015; 19:223–238.5. European Association For The Study Of The Live; European Organisation For Research And Treatment Of Cance. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56:908–943.6. Lee YS, Seo YS, Kim JH, Lee J, Kim HR, Yoo YJ, et al. Can more aggressive treatment improve prognosis in patients with hepatocellular carcinoma? A direct comparison of the Hong Kong liver cancer and Barcelona clinic liver cancer algorithms. Gut Liver. 2018; 12:94–101.7. Kim BH, Lim YS, Kim EY, Kong HJ, Won YJ, Han S, et al. Temporal improvement in survival of patients with hepatocellular carcinoma in a hepatitis B virus-endemic population. J Gastroenterol Hepatol. 2018; 33:475–483.8. Kwak HW, Park JW, Nam BH, Yu A, Woo SM, Kim TH, et al. Clinical outcomes of a cohort series of patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol. 2014; 29:820–829.9. Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol. 2019; 25:93–159.10. Lee KS, Chang HS, Lee SM, Park EC. Economic burden of cancer in Korea during 2000-2010. Cancer Res Treat. 2015; 47:387–398.11. Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010-2016. Clin Mol Hepatol. 2016; 22:7–17.12. Bruix J, Sherman M; Practice Guidelines Committee; American Association for the Study of Liver Disease. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236.13. 13Sheu JC, Sung JL, Chen DS, Yang PM, Lai MY, Lee CS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology. 1985; 89:259–266.14. Kudo M, Kitano M, Sakurai T, Nishida N. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, Nationwide Follow-Up Survey and Clinical Practice Guidelines: the outstanding achievements of the Liver Cancer Study Group of Japan. Dig Dis. 2015; 33:765–770.15. Ueno S, Tanabe G, Nuruki K, Hamanoue M, Komorizono Y, Oketani M, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res. 2002; 24:395–403.16. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999; 19:329–338.17. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015; 35:2155–2166.18. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011; 4:5–10.19. Kudo M. Japan’s successful model of nationwide hepatocellular carcinoma surveillance highlighting the urgent need for global surveillance. Liver Cancer. 2012; 1:141–143.20. Goutté N, Sogni P, Bendersky N, Barbare JC, Falissard B, Farges O. Geographical variations in incidence, management and survival of hepatocellular carcinoma in a Western country. J Hepatol. 2017; 66:537–544.21. Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992-2008. Hepatology. 2012; 55:476–482.22. El -Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011; 365:1118–1127.23. Kudo M, Izumi N, Kubo S, Kokudo N, Sakamoto M, Shiina S, et al. Report of the 20th Nationwide Follow-up Survey of Primary Liver Cancer in Japan. Hepatol Res. 2020; 50:15–46.24. Guarino M, Tortora R, de Stefano G, Coppola C, Morisco F, Salomone Megna A, et al. Adherence to Barcelona Clinic Liver Cancer guidelines in field practice: results of Progetto Epatocarcinoma Campania. J Gastroenterol Hepatol. 2018; 33:1123–1130.25. Leoni S, Piscaglia F, Serio I, Terzi E, Pettinari I, Croci L, et al. Adherence to AASLD guidelines for the treatment of hepatocellular carcinoma in clinical practice: experience of the Bologna Liver Oncology Group. Dig Liver Dis. 2014; 46:549–555.26. Radu P, Groza I, Iancu C, Al Hajjar N, Andreica V, Sparchez Z. Treatment of hepatocellular carcinoma in a tertiary Romanian center. Deviations from BCLC recommendations and influence on survival rate. J Gastrointestin Liver Dis. 2013; 22:291–297.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Yoon et al. Hepatocellular Carcinoma in Korea: an Analysis of the 2015 Korean Nationwide Cancer Registry
- Impact of tumor size on hepatectomy outcomes in hepatocellular carcinoma: a nationwide propensity score matching analysis
- Conditional Survival Estimates Improve Over Time for Patients with Hepatocellular Carcinoma: An Analysis for Nationwide Korea Cancer Registry Database
- The Standization of Clinical Information for the Korean Hepatocellular Carcinoma Registry
- Survival Analysis of Korean Breast Cancer Patients Diagnosed between 1993 and 2002 in Korea: A Nationwide Study of the Cancer Registry