Korean Circ J.
2020 Sep;50(9):773-786. 10.4070/kcj.2020.0031.
Ivabradine for the Therapy of Chronic Stable Angina Pectoris: a Systematic Review and Meta-Analysis
- Affiliations
-
- 1Department of Intensive Care Medicine, Medical Faculty, RWTH Aachen University, Aachen, Germany
- 2Cardiovascular Critical Care & Anesthesia Research and Evaluation (3CARE), Medical Faculty, RWTH Aachen University, Aachen, Germany
- 3Department of Cardiology, Pneumology, Angiology and Intensive Care, Medical Faculty, RWTH Aachen University, Aachen, Germany
- KMID: 2505732
- DOI: http://doi.org/10.4070/kcj.2020.0031
Abstract
- Background and Objectives
Coronary artery disease (CAD) is the number one cause of death worldwide. The If channel inhibitor ivabradine serves as second line medication for the CAD leading symptom angina pectoris. This systematic review and meta-analysis assess the existing evidence of ivabradine in angina pectoris.
Methods
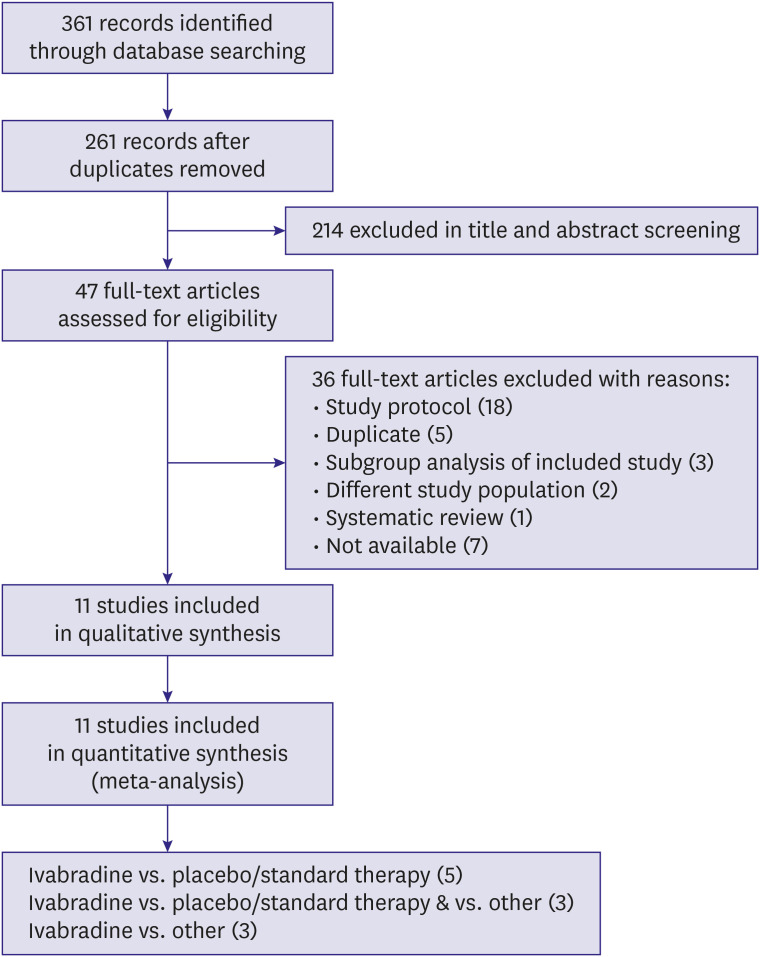
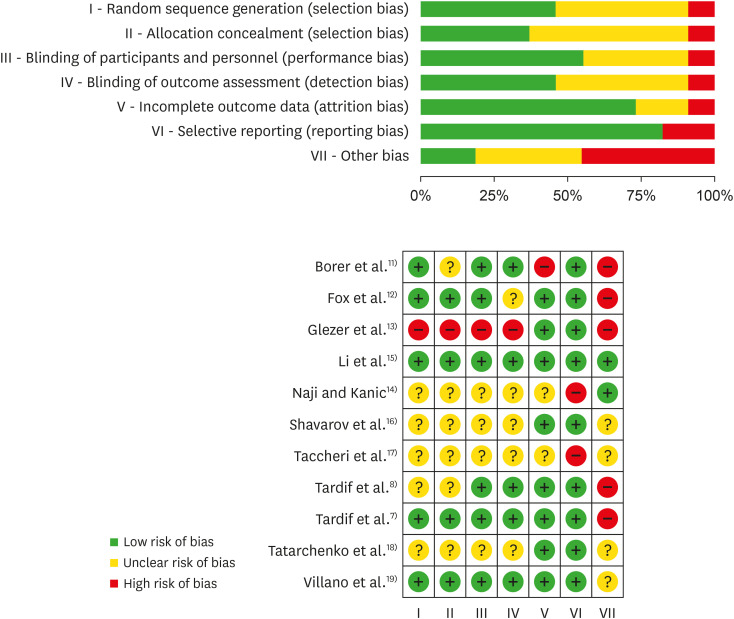
We systematically searched MEDLINE, Embase, CENTRAL, and Web of Science (September 2019) for randomized controlled trials (RCTs) that compared ivabradine versus placebo, standard therapy (ST) or other anti-anginal drugs. Two review authors independently assessed trials for inclusion and performed data extraction. We completed a ‘risk of bias’ assessment for all studies and assessed quality of the trial evidence using the Grading of Recommendations Assessment, Development and Evaluation methodology. We meta-analysed data were applicable and calculated mean differences (MDs) and risk ratios using a random-effects model.
Results
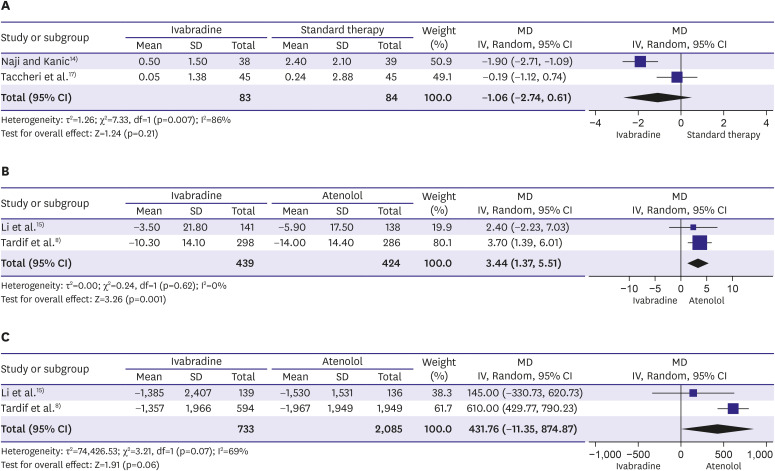
A total of 11 RCTs (n=16,039) were included. Compared to placebo/ST, we found significant effects on the frequency of hospitalisation in a small cohort (n=90; hazard ratio [HR], 0.19; 95% confidence interval [CI], 0.04, −0.92; p=0.04), but no effects on cardiovascular mortality (n=19,102; HR, 1.10; 95% CI, 0.94, 1.28; p=0.25) or the frequency of angina pectoris episodes (n=167; weighted MD, −1.06; 95% CI, −2.74, −0.61; p=0.21).
Conclusions
The present work makes an important contribution to optimal patient care in angina pectoris by complementing the current European Society of Cardiology guideline—recommending class IIa with evidence level B—decisively with 8 further studies.
Figure
Cited by 1 articles
-
Ivabradine for the Therapy of Chronic Stable Angina Pectoris
Soo-Joong Kim
Korean Circ J. 2020;50(9):787-790. doi: 10.4070/kcj.2020.0266.
Reference
-
1. Fox K, Garcia MA, Ardissino D, et al. Guidelines on the management of stable angina pectoris: executive summary: the task force on the management of stable angina pectoris of the European Society of Cardiology. Eur Heart J. 2006; 27:1341–1381. PMID: 16735367.2. Task Force Members. Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013; 34:2949–3003. PMID: 23996286.3. National Clinical Guidelines Centre. Stable angina: methods, evidence & guidance [Internet]. London: National Clinical Guidelines Centre;2011. cited 2020 Jan 24. Available from http://www.nice.org.uk/guidance/cg126/evidence/full-guideline-183176605.4. European Medicines Agency (EMA). Product information ivabradine [Internet]. Amsterdam: European Medicines Agency;2018. cited 2019 Aug 10. Available from https://www.ema.europa.eu/en/documents/product-information/procoralan-epar-product-information_en.pdf.5. Picano E, Alaimo A, Chubuchny V, et al. Noninvasive pacemaker stress echocardiography for diagnosis of coronary artery disease: a multicenter study. J Am Coll Cardiol. 2002; 40:1305–1310. PMID: 12383579.6. Fox K, Ford I, Steg PG, Tendera M, Ferrari R. BEAUTIFUL Investigators. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008; 372:807–816. PMID: 18757088.
Article7. Tardif JC, Ponikowski P, Kahan T. ASSOCIATE Study Investigators. Efficacy of the I(f) current inhibitor ivabradine in patients with chronic stable angina receiving beta-blocker therapy: a 4-month, randomized, placebo-controlled trial. Eur Heart J. 2009; 30:540–548. PMID: 19136486.
Article8. Tardif JC, Ford I, Tendera M, Bourassa MG, Fox K. INITIATIVE Investigators. Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J. 2005; 26:2529–2536. PMID: 16214830.
Article9. Ye L, Ke D, Chen Q, Li G, Deng W, Wu Z. Effectiveness of ivabradine in treating stable angina pectoris. Medicine (Baltimore). 2016; 95:e3245. PMID: 27057864.
Article10. Mengesha HG, Weldearegawi B, Petrucka P, Bekele T, Otieno MG, Hailu A. Effect of ivabradine on cardiovascular outcomes in patients with stable angina: meta-analysis of randomized clinical trials. BMC Cardiovasc Disord. 2017; 17:105. PMID: 28454527.
Article11. Borer JS, Fox K, Jaillon P, Lerebours G. Ivabradine Investigators Group. Antianginal and antiischemic effects of ivabradine, an I(f) inhibitor, in stable angina: a randomized, double-blind, multicentered, placebo-controlled trial. Circulation. 2003; 107:817–823. PMID: 12591750.12. Fox K, Ford I, Steg PG, et al. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014; 371:1091–1099. PMID: 25176136.
Article13. Glezer M, Vasyuk Y, Karpov Y. Efficacy of ivabradine in combination with beta-blockers versus uptitration of beta-blockers in patients with stable angina (CONTROL-2 study). Adv Ther. 2018; 35:341–352. PMID: 29508153.
Article14. Naji F, Kanic V. Pretreatment with ivabradine reduces periprocedural myocardial injury and infarction in stable ischemic patients undergoing coronary intervention. Eur Heart J. 2014; 35:127–128.15. Li Y, Jing L, Li Y, et al. The efficacy and safety of ivabradine hydrochloride versus atenolol in Chinese patients with chronic stable angina pectoris. Pharmacoepidemiol Drug Saf. 2014; 23:1183–1191. PMID: 24757084.
Article16. Shavarov AA, Kiiakbaev GK, Kobalava ZhD. A possibility to interchange heart rate-slowing therapy with ivabradine and atenolol in patients with stable angina pectoris. Ter Arkh. 2013; 85:77–83.17. Taccheri T, Calcagno S, Carnesale R, et al. Comparison of the efficacy and tolerability of ivabradine vs ranolazine vs standard medical therapy in patients of chronic stable angina pectoris despite coronary percutaneous revascularization. Eur Heart J. 2014; 35:323.18. Tatarchenko IP, Pozdniakova NV, Biriuchenko MV. Comparative efficacy of ivabradine and atenolol in correction of clinical and functional parameters in patients with stable effort angina. Kardiologiia. 2008; 48:60–61.19. Villano A, Di Franco A, Nerla R, et al. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol. 2013; 112:8–13. PMID: 23558043.
Article20. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [Internet]. London: The Cochrane Collaboration;2011. cited 2018 Jul 6. Available from http://www.cochrane-handbook.org.21. The Cochrane Collaboration. Review Manager (RevMan). 5.0. Copenhagen: The Cochrane Collaboration;2008.22. Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995; 25:333–341. PMID: 7829785.
Article23. EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990; 16:199–208. PMID: 10109801.24. European Medicines Agency. PRAC recommends measures to reduce risk of heart problems with Corlentor/Procoralan (ivabradine) [Internet]. Amsterdam: European Medicines Agency;2014. cited 2019 Jul 10. Available from https://www.ema.europa.eu/en/news/prac-recommends-measures-reduce-risk-heart-problems-corlentorprocoralan-ivabradine.25. Kaski JC, Gloekler S, Ferrari R, et al. Role of ivabradine in management of stable angina in patients with different clinical profiles. Open Heart. 2018; 5:e000725. PMID: 29632676.
Article26. Werdan K, Perings S, Köster R, et al. Effectiveness of ivabradine treatment in different subpopulations with stable angina in clinical practice: a pooled analysis of observational studies. Cardiology. 2016; 135:141–150. PMID: 27333284.
Article27. Fox K, Ford I, Steg PG, et al. Relationship between ivabradine treatment and cardiovascular outcomes in patients with stable coronary artery disease and left ventricular systolic dysfunction with limiting angina: a subgroup analysis of the randomized, controlled BEAUTIFUL trial. Eur Heart J. 2009; 30:2337–2345. PMID: 19720635.
Article28. Borer JS, Swedberg K, Komajda M, et al. Efficacy profile of ivabradine in patients with heart failure plus angina pectoris. Cardiology. 2017; 136:138–144. PMID: 27614723.
Article29. Amosova E, Andrejev E, Zaderey I, Rudenko U, Ceconi C, Ferrari R. Efficacy of ivabradine in combination with beta-blocker versus uptitration of beta-blocker in patients with stable angina. Cardiovasc Drugs Ther. 2011; 25:531–537. PMID: 21830063.
Article30. Ferrari R, Camici PG, Crea F, et al. Expert consensus document: a ‘diamond’ approach to personalized treatment of angina. Nat Rev Cardiol. 2018; 15:120–132. PMID: 28880025.