J Korean Med Sci.
2020 Aug;35(31):e287. 10.3346/jkms.2020.35.e287.
Viral Load Kinetics of SARS-CoV-2Infection in Saliva in Korean Patients:a Prospective Multi-center Comparative Study
- Affiliations
-
- 1Department of Infectious Diseases, Chonnam National University Hospital, Gwangju, Korea
- 2Department of Infectious Diseases, Keimyung University Dongsan Hospital, Daegu, Korea
- 3Department of Infectious Diseases, Chonnam National University Bitgoeul Hospital, Gwangju, Korea
- 4Department of Infectious Diseases, Chonnam National University Hwasun Hospital, Hwasun, Korea
- 5Department of Laboratory Medicine, Chonnam National University Hospital, Gwangju, Korea
- KMID: 2505201
- DOI: http://doi.org/10.3346/jkms.2020.35.e287
Abstract
- Background
This study was performed to compare the viral load and kinetics of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in saliva with those in standard nasopharyngeal/oropharyngeal (NP/OP) swabs.
Methods
Fifteen patients with SARS-CoV-2 infection from four hospitals were prospectively enrolled and matched samples of nasopharyngeal/oropharyngeal swabs and saliva were collected at Day 1 of admission and every other day till consequently negative for two times. Real-time reverse transcription polymerase chain reaction (rRT-PCR) was performed to detect the envelope (E) and RNA-dependent RNA polymerase (RdRP) genes.
Results
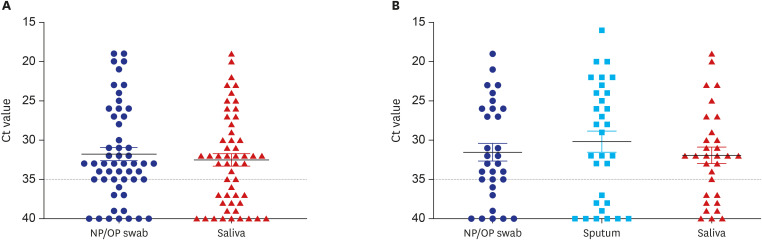
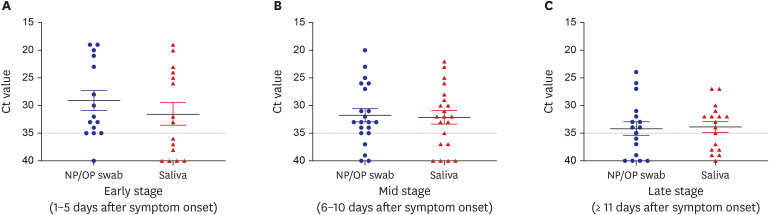
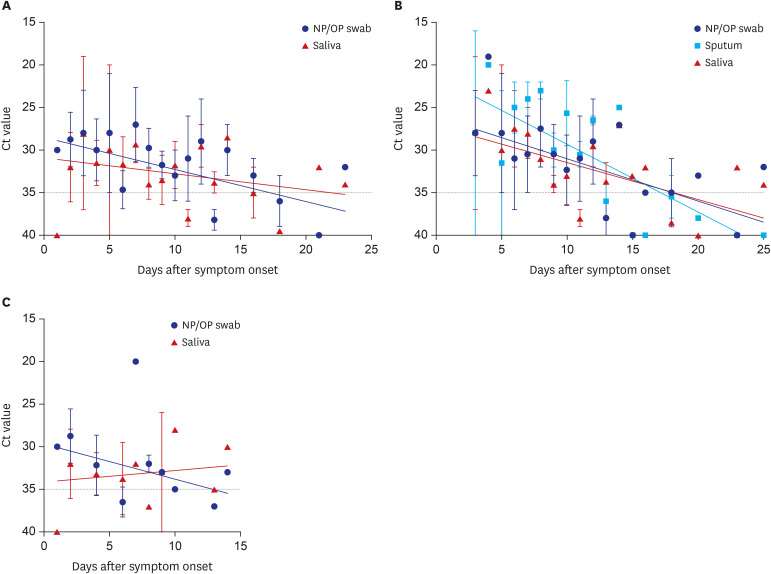
The cycle threshold values of saliva were comparable to those of NP/OP swabs overall (P = 0.720, Mann–Whitney U test). However, the overall sensitivity of rRT-PCR using saliva was 64% (34/53), which is lower than the 77% (41/53) using NP/OP swabs. The sensitivity of rRT-PCR using saliva was especially lower in early stage of symptom onset (1–5 days; 8/15; 53%) and in patients who did not have sputum (12/22; 55%).
Conclusion
Saliva sample itself is not appropriate for initial diagnosis of coronavirus disease 2019 (COVID-19) to replace NP/OP swabs, especially for the person who does not produce sputum. COVID-19 cannot be excluded when the test using saliva is negative, and it is necessary to retest using NP/OP swabs.
Figure
Cited by 1 articles
-
Serial Screening for SARS-CoV-2 in Rectal Swabs of Symptomatic COVID-19 Patients
Sung Hoon Jung, Sei Won Kim, Heayon Lee, Jung Hwan Oh, Jihyang Lim
J Korean Med Sci. 2021;36(44):e301. doi: 10.3346/jkms.2021.36.e301.
Reference
-
1. U.S. Food and Drug Administration. Accelerated emergency use authorization (EUA) summary. SARS-CoV-2 assay (Rutgers Clinical Genomics Laboratory). Updated 2020. Accessed August 5, 2020. https://www.fda.gov/media/136875/download.2. Azzi L, Carcano G, Gianfagna F, Grossi P, Gasperina DD, Genoni A, et al. Saliva is a reliable tool to detect SARS-CoV-2. J Infect. 2020; 81(1):e45–50. PMID: 32298676.
Article3. Pasomsub E, Watcharananan SP, Boonyawat K, Janchompoo P, Wongtabtim G, Suksuwan W, et al. Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: a cross-sectional study. Clin Microbiol Infect. Forthcoming. 2020; DOI: 10.1016/j.cmi.2020.05.001.
Article4. Tu YP, Jennings R, Hart B, Cangelosi G, Wood R, Wehber K, et al. Patient-collected tongue, nasal, and mid-turbinate swabs for SARS-CoV-2 yield equivalent sensitivity to health care worker collected nasopharyngeal swabs. medRxiv. Updated 2020. Accessed August 5, 2020. DOI: 10.1101/2020.04.01.20050005.5. Wyllie AL, Fournier J, Casanovas-Massana A, Campbell M, Tokuyama M, Vijayakumar P, et al. Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs. medRxiv. Updated 2020. Accessed August 5, 2020. DOI: 10.1101/2020.04.16.20067835.6. Becker D, Sandoval E, Amin A, Hoff PD, Diets A, Leonetti N, et al. Saliva is less sensitive than nasopharyngeal swabs for COVID-19 detection in the community setting. medRxiv. Updated 2020. Accessed August 5, 2020. DOI: 10.1101/2020.05.11.20092338.7. Yoon JG, Yoon J, Song JY, Yoon SY, Lim CS, Seong H, et al. Clinical significance of a high SARS-CoV-2 viral load in the saliva. J Korean Med Sci. 2020; 35(20):e195. PMID: 32449329.
Article8. Jamal AJ, Mozafarihashjin M, Coomes E, Powis J, Li AX, Paterson A, et al. Sensitivity of nasopharyngeal swabs and saliva for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. Forthcoming. 2020; DOI: 10.1093/cid/ciaa848.
Article9. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020; 323(18):1843–1844.
Article10. Pan Y, Zhang D, Yang P, Poon LL, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020; 20(4):411–412. PMID: 32105638.
Article11. Kim YG, Yun SG, Kim MY, Park K, Cho CH, Yoon SY, et al. Comparison between saliva and nasopharyngeal swab specimens for detection of respiratory viruses by multiplex reverse transcription-PCR. J Clin Microbiol. 2016; 55(1):226–233. PMID: 27807150.
Article12. To KK, Yip CC, Lai CY, Wong CK, Ho DT, Pang PK, et al. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin Microbiol Infect. 2019; 25(3):372–378. PMID: 29906597.
Article13. To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020; 20(5):565–574. PMID: 32213337.
Article14. To KK, Tsang OT, Yip CC, Chan KH, Wu TC, Chan JM, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020; 71(15):841–843. PMID: 32047895.
Article15. Williams E, Bond K, Zhang B, Putland M, Williamson DA. Saliva as a non-invasive specimen for detection of SARS-CoV-2. J Clin Microbiol. 2020; 58(8):e00776-20. PMID: 32317257.
Article16. Chen JH, Yip CC, Poon RW, Chan KH, Cheng VC, Hung IF, et al. Evaluating the use of posterior oropharyngeal saliva in a point-of-care assay for the detection of SARS-CoV-2. Emerg Microbes Infect. 2020; 9(1):1356–1359. PMID: 32459137.
Article17. Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020; 581(7809):465–469. PMID: 32235945.
Article18. Kim JY, Ko JH, Kim Y, Kim YJ, Kim JM, Chung YS, et al. Viral load kinetics of SARS-CoV-2 infection in first two patients in Korea. J Korean Med Sci. 2020; 35(7):e86. PMID: 32080991.
Article19. Kim ES, Chin BS, Kang CK, Kim NJ, Kang YM, Choi JP, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID-19. J Korean Med Sci. 2020; 35(13):e142. PMID: 32242348.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Viral Load Kinetics of SARS-CoV-2 Infection in First Two Patients in Korea
- Clinical Significance of a High SARS-CoV-2 Viral Load in the Saliva
- Viral Shedding Kinetics in Patients with COVID-19
- Reinfection of SARS-CoV-2 Variants in Immunocompromised Patients with Prolonged or Relapsed Viral Shedding
- Replication kinetics and infectivity of SARS-CoV-2 Omicron variant sublineages recovered in the Republic of Korea