Anesth Pain Med.
2020 Jul;15(3):283-290. 10.17085/apm.20010.
Bleeding properties according to surgical sites during pediatric craniotomy: a retrospective study comparing the two stages of epilepsy surgery
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Severance Hospital and Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Anesthesiology, Muhimbili University of Health and Allied Sciences, Dar Es Salaam, United Republic of Tanzania
- KMID: 2504889
- DOI: http://doi.org/10.17085/apm.20010
Abstract
- Background
During pediatric epilepsy surgery, due to low circulating blood volume, intraoperative bleeding can result in significant hemodynamic instability, thereby requiring meticulous hemodynamic and transfusion strategies. Knowing the source of bleeding during the procedure would allow medical staff to better prepare the perioperative protocols for these patients. We compared intraoperative bleeding between the first (involving skin to meninges) and second (involving brain parenchyma) stages of epilepsy surgery to investigate the differences between various anatomical sites.
Methods
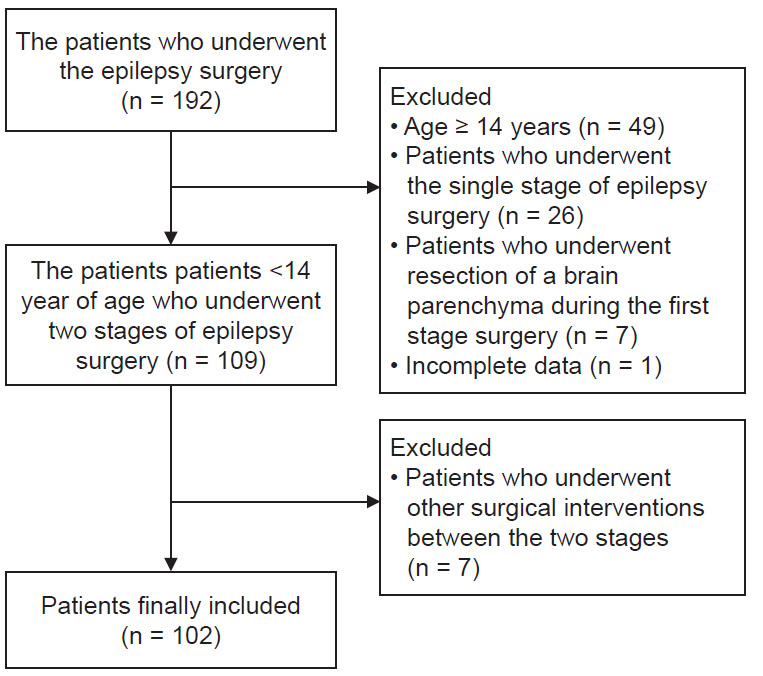
We reviewed the electronic medical records of 102 pediatric patients < 14 years old who underwent two-stage epilepsy surgeries during January 2012–2016. Invasive subdural grids were placed via craniotomy during Stage 1 and the epileptogenic zone was removed during Stage 2 of the surgery. We compared the volume of intraoperative bleeding between these two surgeries and identified variables associated with bleeding using multivariate regression analysis.
Results
Both surgeries resulted in similar intraoperative bleeding (24 vs. 26 ml/kg, P = 0.835), but Stage 2 required greater volumes of blood transfusion than Stage 1 (18.4 vs. 14.8 ml/kg, P = 0.011). Massive bleeding was associated with patients < 7 years old in Stage 1 and weighing < 18 kg in Stage 2.
Conclusions
The volume of intraoperative bleeding was similar between the two stages of pediatric epilepsy surgery and was large enough to require blood transfusions. Thus, blood loss during pediatric epilepsy surgery occurred at both anatomic sites. This indicates the necessity of early preparation for blood transfusion in both stages of pediatric epilepsy surgery.
Figure
Reference
-
1. Vassal O, Desgranges FP, Tosetti S, Burgal S, Dailler F, Javouhey E, et al. Risk factors for intraoperative allogeneic blood transfusion during craniotomy for brain tumor removal in children. Paediatr Anaesth. 2016; 26:199–206.2. Keung CY, Smith KR, Savoia HF, Davidson AJ. An audit of transfusion of red blood cell units in pediatric anesthesia. Paediatr Anaesth. 2009; 19:320–8.3. Kellermann TS, Wagner JL, Smith G, Karia S, Eskandari R. Surgical management of pediatric epilepsy: decision-making and outcomes. Pediatr Neurol. 2016; 64:21–31.4. Guan J, Karsy M, Ducis K, Bollo RJ. Surgical strategies for pediatric epilepsy. Transl Pediatr. 2016; 5:55–66.5. Roth J, Carlson C, Devinsky O, Harter DH, Macallister WS, Weiner HL. Safety of staged epilepsy surgery in children. Neurosurgery. 2014; 74:154–62.6. Bingaman WE, Bulacio J. Placement of subdural grids in pediatric patients: technique and results. Childs Nerv Syst. 2014; 30:1897–904.7. Irita K. Risk and crisis management in intraoperative hemorrhage: human factors in hemorrhagic critical events. Korean J Anesthesiol. 2011; 60:151–60.8. Goobie SM, Haas T. Perioperative bleeding management in pediatric patients. Curr Opin Anaesthesiol. 2016; 29:352–8.9. Goobie SM, Meier PM, Pereira LM, McGowan FX, Prescilla RP, Scharp LA, et al. Efficacy of tranexamic acid in pediatric craniosynostosis surgery: a double-blind, placebo-controlled trial. Anesthesiology. 2011; 114:862–71.10. Yin J, Tian L. Joint confidence region estimation for area under ROC curve and Youden index. Stat Med. 2014; 33:985–1000.11. Schmotzer CL, Brown AE, Roth S, Johnson J, Ines-Castillejo M, Reisner A, et al. Procedure-specific preoperative red blood cell preparation and utilization management in pediatric surgical patients. Transfusion. 2010; 50:861–7.12. Cholette JM, Faraoni D, Goobie SM, Ferraris V, Hassan N. Patient blood management in pediatric cardiac surgery: a review. Anesth Analg. 2018; 127:1002–16.13. Lee JW. Transfusion guidelines in pediatric patients. Anesth Pain Med. 2015; 10:141–8.14. Aljaaly HA, Aldekhayel SA, Diaz-Abele J, Karunanayka M, Gilardino MS. Effect of erythropoietin on transfusion requirements for craniosynostosis surgery in children. J Craniofac Surg. 2017; 28:1315–9.15. Mantadakis E. Advances in pediatric intravenous iron therapy. Pediatr Blood Cancer. 2016; 63:11–6.16. Shiek Ahmad B, O'Brien TJ, Gorelik A, Hill KD, Wark JD. Bone mineral changes in epilepsy patients during initial years of antiepileptic drug therapy. J Clin Densitom. 2016; 19:450–6.17. Kurahashi H, Takami A, Murotani K, Numoto S, Okumura A. Decreased platelet count in children with epilepsy treated with valproate and its relationship to the immature platelet fraction. Int J Hematol. 2018; 107:105–11.18. Finsterer J, Pelzl G, Hess B. Severe, isolated thrombocytopenia under polytherapy with carbamazepine and valproate. Psychiatry Clin Neurosci. 2001; 55:423–6.19. Holtzer CD, Reisner-Keller LA. Phenytoin-induced thrombocytopenia. Ann Pharmacother. 1997; 31:435–7.20. Cannizzaro E, Albisetti M, Wohlrab G, Schmugge M. Severe bleeding complications during antiepileptic treatment with valproic acid in children. Neuropediatrics. 2007; 38:42–5.21. Gerstner T, Teich M, Bell N, Longin E, Dempfle CE, Brand J, et al. Valproate-associated coagulopathies are frequent and variable in children. Epilepsia. 2006; 47:1136–43.22. Kreuz W, Linde R, Funk M, Meyer-Schrod R, Föll E, Nowak-Göttl U, et al. Valproate therapy induces von Willebrand disease type I. Epilepsia. 1992; 33:178–84.23. Gidal B, Spencer N, Maly M, Pitterle M, Williams E, Collins M, et al. Valproate-mediated disturbances of hemostasis: relationship to dose and plasma concentration. Neurology. 1994; 44:1418–22.24. Manohar C, Avitsian R, Lozano S, Gonzalez-Martinez J, Cata JP. The effect of antiepileptic drugs on coagulation and bleeding in the perioperative period of epilepsy surgery: the Cleveland Clinic experience. J Clin Neurosci. 2011; 18:1180–4.25. Triplett DA. Coagulation and bleeding disorders: review and update. Clin Chem. 2000; 46(8 Pt 2):1260–9.26. Jackson SP, Nesbitt WS, Kulkarni S. Signaling events underlying thrombus formation. J Thromb Haemost. 2003; 1:1602–12.27. Morotti A, Charidimou A, Phuah CL, Jessel MJ, Schwab K, Ayres AM, et al. Association between serum calcium level and extent of bleeding in patients with intracerebral hemorrhage. JAMA Neurol. 2016; 73:1285–90.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Applications and Outcomes of Epilepsy Surgery in Pediatric Population
- Endoscopic Treatment of Hypothalamic Hamartomas
- Sleep and Epilepsy in Clinical Practice "fears, regas, deliria, leaps out of bed and seizures during the night" - Hippocrates
- Stereoelectroencephalography in Pediatric Epilepsy Surgery
- Surgical Candidates for Intractable Pediatric Epilepsy