Ann Surg Treat Res.
2020 Aug;99(2):97-109. 10.4174/astr.2020.99.2.97.
Oncologic effects of adjuvant chemotherapy in patientswith ypT0–2N0 rectal cancer after neoadjuvantchemoradiotherapy and curative surgery: a meta-analysis
- Affiliations
-
- 1Research Institute of Clinical Medicine of Jeonbuk National University-Biomedical Research Institute of Jeonbuk National University Hospital, Jeonju, Korea
- KMID: 2504822
- DOI: http://doi.org/10.4174/astr.2020.99.2.97
Abstract
- Purpose
The role of adjuvant chemotherapy for patients with ypT0–2N0 rectal cancer following neoadjuvantchemoradiotherapy (nCRT) and curative surgery is uncertain. We performed a meta-analysis using selected studies tocompare adjuvant chemotherapy with observation for this cohort of patients.
Methods
PubMed, Embase, and the Cochrane Library were searched. Data were pooled, and overall effect size wascalculated using random effect models. Outcome measures were 5-year overall survival (OS), disease-free survival (DFS),local, and distant recurrence.
Results
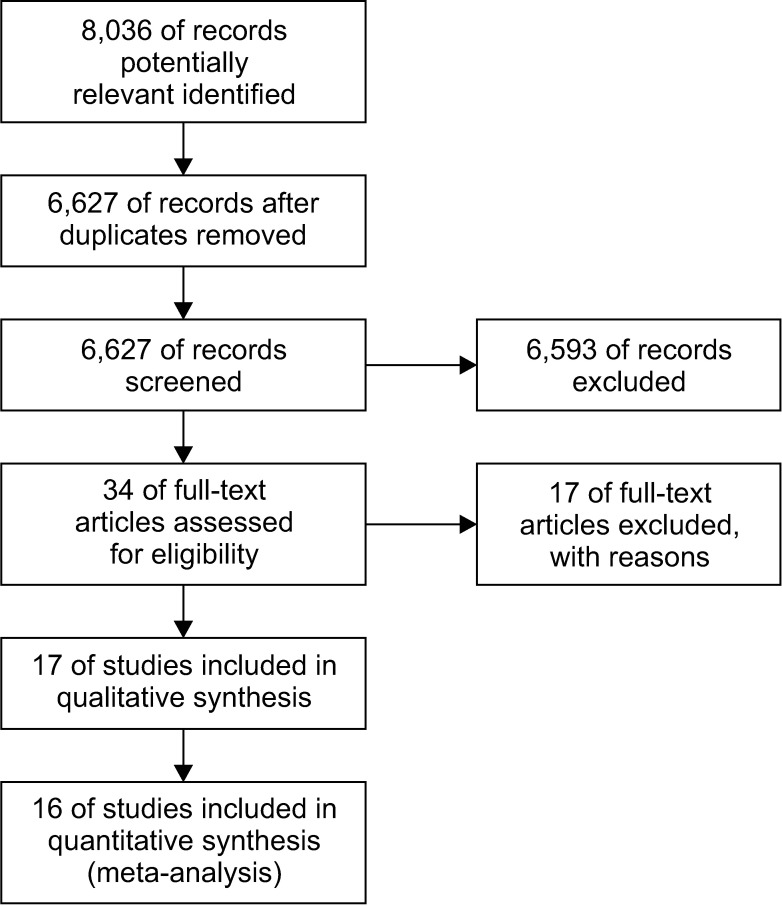
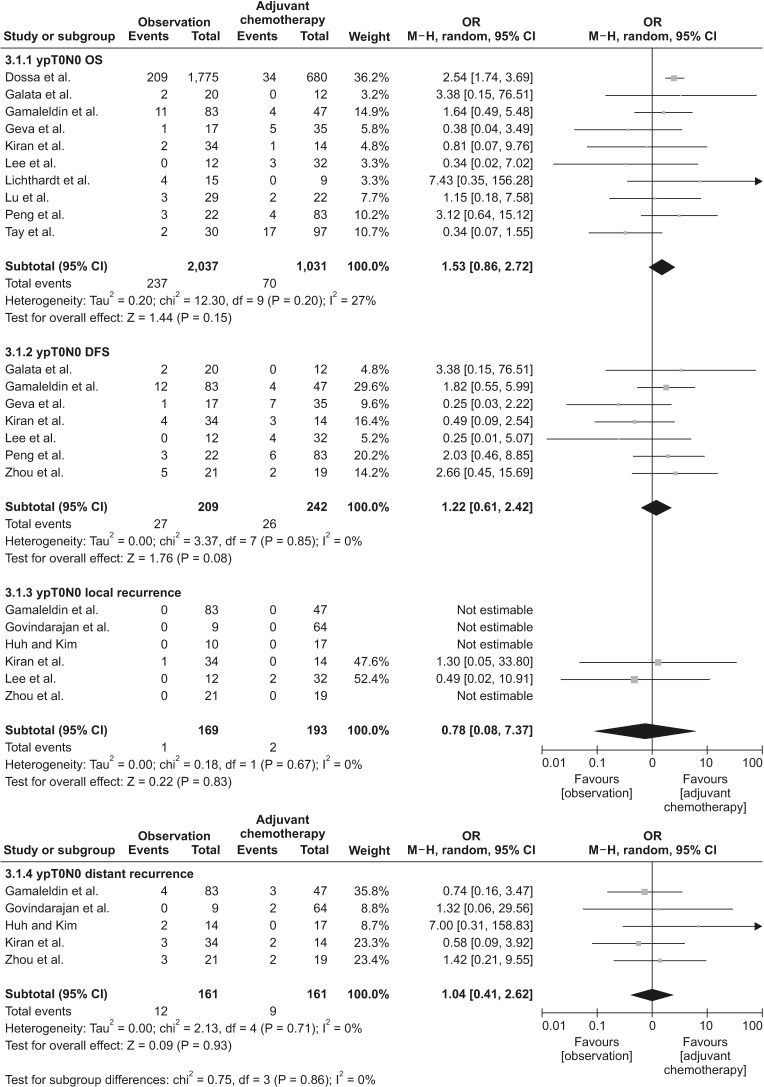
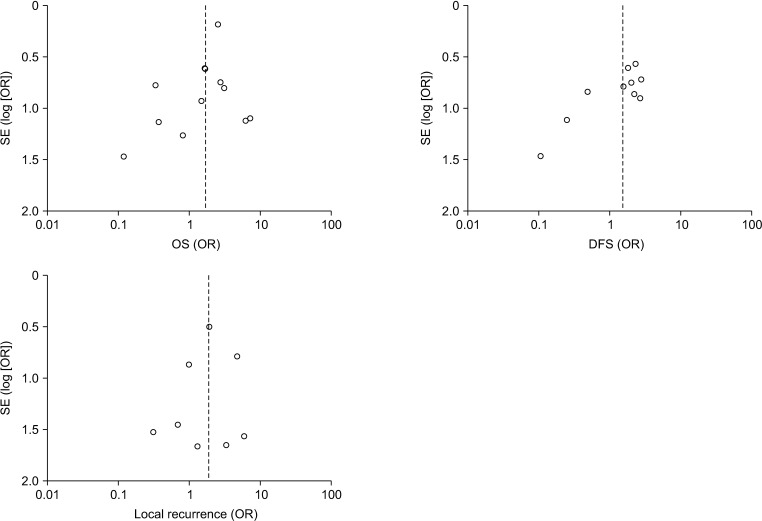
We included 17 nonrandomized studies for qualitative analysis and 16 nonrandomized studies that examined 4,747patients for the meta-analysis. In analysis of patients with ypT0N0 rectal cancer, adjuvant chemotherapy had no significanteffect on OS (odds ratio [OR], 1.53; 95% confidence interval [CI], 0.86–2.72; I2 = 27%), DFS (OR, 1.22; 95% CI, 0.61–2.42; I2 =5%), local recurrence (OR, 0.78; 95% CI, 0.08–7.37; I2 = 0%), and distant recurrence (OR, 1.04; 95% CI, 0.41–2.62; I2 = 0%).In analysis of patients with ypT1–2N0 rectal cancer, adjuvant chemotherapy also had no significant effect on OS (OR, 2.15;95% CI, 0.59–7.80; I2 = 26%), DFS (OR, 1.66; 95% CI, 0.35–7.85; I2 = 44%), local recurrence (OR, 2.56; 95% CI, 0.72–9.13; I2 =0%), and distant recurrence (OR, 1.15; 95% CI, 0.23–5.87; I2 = 0%).
Conclusion
Adjuvant chemotherapy may have no oncologic benefits in patients with ypT0–2N0 rectal cancer after nCRTand radical surgery. Routine use of adjuvant chemotherapy for those patients may be avoided.
Figure
Reference
-
1. Scott NA, Susnerwala S, Gollins S, Myint AS, Levine E. Preoperative neoadjuvant therapy for curable rectal cancer: reaching a consensus 2008. Colorectal Dis. 2009; 11:245–248. PMID: 18637934.2. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012; 30:1926–1933. PMID: 22529255.3. van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomized controlled TME trial. Lancet Oncol. 2011; 12:575–582. PMID: 21596621.4. Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014; 15:184–190. PMID: 24440473.5. Breugom AJ, van Gijn W, Muller EW, Berglund Å, van den Broek CB, Fokstuen T, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. 2015; 26:696–701. PMID: 25480874.6. Glynne-Jones R, Counsell N, Quirke P, Mortensen N, Maraveyas A, Meadows HM, et al. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. 2014; 25:1356–1362. PMID: 24718885.7. Huh JW, Kim HR. Postoperat ive chemotherapy af ter neoadjuvant chemoradiation and surgery for rectal cancer: is it essential for patients with ypT0-2N0. J Surg Oncol. 2009; 100:387–391. PMID: 19582821.8. Lee KH, Kim JC, Kim JY, Kim JS. Oncologic results and prognost ic predictors of pat ients with local ly advanced rectal cancer showing ypN0 af ter radical surgery following neoadjuvant chemoradiotherapy. Int J Colorectal Dis. 2015; 30:1041–1050. PMID: 26002751.9. Park IJ, Kim DY, Kim HC, Kim NK, Kim HR, Kang SB, et al. Role of adjuvant chemotherapy in ypT0-2N0 patients treated with preoperative chemoradiation therapy and radical resection for rectal cancer. Int J Radiat Oncol Biol Phys. 2015; 92:540–547. PMID: 26068489.10. Petrelli F, Coinu A, Lonati V, Barni S. A systematic review and meta-analysis of adjuvant chemotherapy after neoadjuvant treatment and surgery for rectal cancer. Int J Colorectal Dis. 2015; 30:447–457. PMID: 25433820.11. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. Ann Intern Med. 2009; 151:264–269. W64PMID: 19622511.12. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions, version 5.1.0. Chichester: Wiley;2011.13. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses [Internet]. Ottawa: Ottawa Hospital Research Institute;c2019. cited 2019 Jul 1. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.14. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560. PMID: 12958120.15. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–188. PMID: 3802833.16. Thabane L, Akhtar-Danesh N. Guidelines for reporting descriptive statistics in health research. Nurse Res. 2008; 15:72–81.17. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensit ivit y of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008; 37:1148–1157. PMID: 18424475.18. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634. PMID: 9310563.19. Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000; 320:1574–1577. PMID: 10845965.20. Dossa F, Acuna SA, Rickles AS, Berho M, Wexner SD, Quereshy FA, et al. Association between adjuvant chemotherapy and overall survival in patients with rectal cancer and pathological complete response after neoadjuvant chemotherapy and resection. JAMA Oncol. 2018; 4:930–937. PMID: 29710274.21. Galata C, Merx K, Mai S, Gaiser T, Wenz F, Post S, et al. Impact of adjuvant chemotherapy on patients with ypT0-2 ypN0 rectal cancer after neoadjuvant chemoradiation: a cohort study from a tertiary referral hospital. World J Surg Oncol. 2018; 16:156. PMID: 30071852.22. Lu Z, Cheng P, Zhang MG, Wang XS, Zheng ZX. Is adjuvant chemotherapy necessary for patients with ypT0-2N0 rectal cancer treated with neoadjuvant chemoradiotherapy and curative surgery? Gastroenterol Rep (Oxf). 2018; 6:277–283. PMID: 30430016.23. Peng JH, Lin JZ, Rong YM, Zhu Y, Deng YX, Zhao YJ, et al. Oxaliplatin-containing adjuvant chemotherapy improves the survival of locally advanced rectal cancer patients with pathological complete response after pre-operative chemoradiotherapy. Gastroenterol Rep (Oxf). 2018; 6:195–201. PMID: 30151204.24. Gamaleldin M, Church JM, Stocchi L, Kalady M, Liska D, Gorgun E. Is routine use of adjuvant chemotherapy for rectal cancer with complete pathological response justified. Am J Surg. 2017; 213:478–483. PMID: 27939008.25. Lichthardt S, Zenorini L, Wagner J, Baur J, Kerscher A, Matthes N, et al. Impact of adjuvant chemotherapy after neoadjuvant radio- or radiochemotherapy for patients with locally advanced rectal cancer. J Cancer Res Clin Oncol. 2017; 143:2363–2373. PMID: 28756493.26. Tay RY, Jamnagerwalla M, Steel M, Wong HL, McKendrick JJ, Faragher I, et al. Survival impact of adjuvant chemotherapy for resected locally advanced rectal adenocarcinoma. Clin Colorectal Cancer. 2017; 16:e45–e54. PMID: 27825672.27. Geva R, Itzkovich E, Shamai S, Shacham-Shmueli E, Soyfer V, Klausner JM, et al. Is there a role for adjuvant chemotherapy in pathological complete response rectal cancer tumors following neoadjuvant chemoradiotherapy? J Cancer Res Clin Oncol. 2014; 140:1489–1494. PMID: 24849731.28. You KY, Huang R, Ding PR, Qiu B, Zhou GQ, Chang H, et al. Selective use of adjuvant chemotherapy for rectal cancer patients with ypN0. Int J Colorectal Dis. 2014; 29:529–538. PMID: 24474499.29. Kiran RP, Kirat HT, Burgess AN, Nisar PJ, Kalady MF, Lavery IC. Is adjuvant chemotherapy really needed after curative surgery for rectal cancer patients who are node-negative after neoadjuvant chemoradiotherapy? Ann Surg Oncol. 2012; 19:1206–1212. PMID: 21935748.30. Zhou J, Qiu H, Lin G, Xiao Y, Wu B, Wu W, et al. Is adjuvant chemotherapy necessary for patients with pathological complete response after neoadjuvant chemoradiotherapy and radical surgery in locally advanced rectal cancer? Long-term analysis of 40 ypCR patients at a single center. Int J Colorectal Dis. 2016; 31:1163–1168. PMID: 27044403.31. Jung KU, Kim HC, Park JO, Park YS, Park HC, Choi DH, et al. Adjuvant chemotherapy af ter neoadjuvant chemoradiation and curative resection for rectal cancer: is it necessary for all patients? J Surg Oncol. 2015; 111:439–444. PMID: 25488390.32. Gao P, Song YX, Sun JX, Chen XW, Xu YY, Zhao JH, et al. Which is the best postoperative chemotherapy regimen in patients with rectal cancer after neoadjuvant therapy. BMC Cancer. 2014; 14:888. PMID: 25428401.33. Govindarajan A, Reidy D, Weiser MR, Paty PB, Temple LK, Guillem JG, et al. Recurrence rates and prognostic factors in ypN0 rectal cancer after neoadjuvant chemoradiation and total mesorectal excision. Ann Surg Oncol. 2011; 18:3666–3672. PMID: 21590450.34. Taal BG, Van Tinteren H, Zoetmulder FA. NACCP group. Adjuvant 5FU plus levamisole in colonic or rectal cancer: improved survival in stage II and III. Br J Cancer. 2001; 85:1437–1443. PMID: 11720425.35. Twelves C, Wong A, Nowacki MP, Abt M, Burris H 3rd, Carrato A, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005; 352:2696–2704. PMID: 15987918.36. André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplat in, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009; 27:3109–3116. PMID: 19451431.37. Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. 2007; 25:4379–4386. PMID: 17906203.38. De Stefano A, Moretto R, Bucci L, Pepe S, Romano FJ, Cella AC, et al. Adjuvant treatment for locally advanced rectal cancer patients after preoperative chemoradiotherapy: when, and for whom. Clin Colorectal Cancer. 2014; 13:185–191. PMID: 25080847.39. Haynes AB, You YN, Hu CY, Eng C, Kopetz ES, Rodriguez-Bigas MA, et al. Postoperative chemotherapy use after neoadjuvant chemoradiotherapy for rectal cancer: analysis of Surveillance, Epidemiology, and End Results-Medicare data, 1998-2007. Cancer. 2014; 120:1162–1170. PMID: 24474245.40. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006; 355:1114–1123. PMID: 16971718.41. Kim TH, Chang HJ, Kim DY, Jung KH, Hong YS, Kim SY, et al. Pathologic nodal classification is the most discriminating prognostic factor for disease-free survival in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Int J Radiat Oncol Biol Phys. 2010; 77:1158–1165. PMID: 19800178.42. Kuo LJ, Liu MC, Jian JJ, Horng CF, Cheng TI, Chen CM, et al. Is final TNM staging a predictor for survival in locally advanced rectal cancer after preoperative chemoradiation therapy? Ann Surg Oncol. 2007; 14:2766–2772. PMID: 17551794.43. Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010; 11:835–844. PMID: 20692872.44. de Campos-Lobato LF, Stocchi L, da Luz Moreira A, Geisler D, Dietz DW, Lavery IC, et al. Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann Surg Oncol. 2011; 18:1590–1598. PMID: 21207164.45. Chang GJ, Park IJ, Eng C, You YN, Kopetz S, Overman MJ, et al. Exploratory analysis of adjuvant chemotherapy benefits after preoperative chemoradiotherapy and radical resection for rectal cancer. J Clin Oncol. 2012; 30(15 Suppl):3556.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Outcomes of Ileostomy Closure According to Timing During Adjuvant Chemotherapy After Rectal Cancer Surgery
- Update of Adjuvant Chemotherapy for Resected Gastric Cancer
- Role of Adjuvant Radiotherapy in Gastric Cancer
- Oncologic outcomes of early adjuvant chemotherapy initiation in patients with stage III colon cancer
- Organ Preservation Strategies After Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer