Ann Surg Treat Res.
2015 Sep;89(3):124-130. 10.4174/astr.2015.89.3.124.
Oncologic outcomes of early adjuvant chemotherapy initiation in patients with stage III colon cancer
- Affiliations
-
- 1Department of Surgery, Keimyung University Dongsan Medical Center, Daegu, Korea. sgbeak@dsmc.or.kr
- KMID: 2166936
- DOI: http://doi.org/10.4174/astr.2015.89.3.124
Abstract
- PURPOSE
Although adjuvant chemotherapy reduces the risk of disease recurrence in stage III colon cancer patients, published guidelines do not specify when it should be initiated. This study aimed to assess the effect of adjuvant chemotherapy initiation time on disease recurrence and survival in stage III colon cancer patients undergoing curative surgical resection.
METHODS
The medical records of stage III colon cancer patients undergoing curative resection between February 2004 and December 2009 were reviewed.
RESULTS
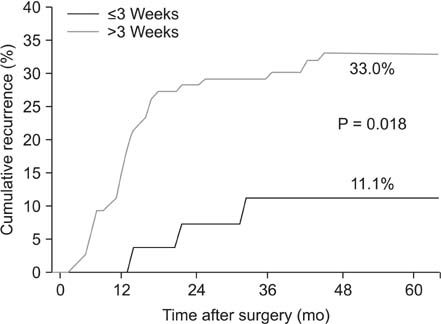
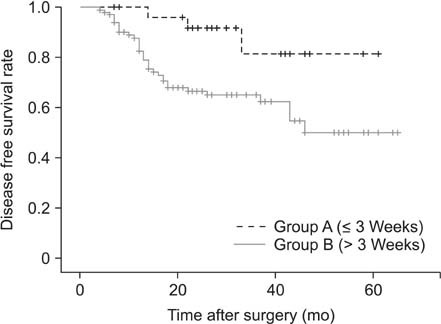
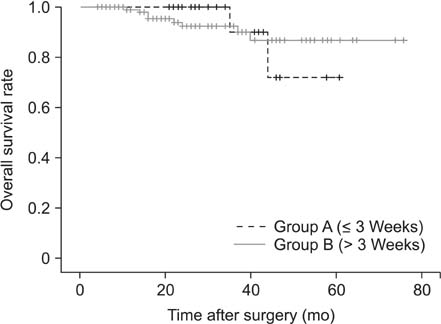
Of the 133 enrolled patients, 27 (20.3%) began adjuvant chemotherapy within 3 weeks of surgery, whereas 106 (79.7%) did after 3 weeks following surgery. Patients receiving chemotherapy within 3 weeks of surgery were less likely to experience recurrences than those beginning treatment later (11.1% vs. 33%, P = 0.018). The mean disease-free survival of patients receiving adjuvant therapy earlier was 54.6 months, whereas that of patients with later treatment was 43.5 months (P = 0.014). However, no significant differences in overall survival were observed between the 2 groups.
CONCLUSION
Adjuvant chemotherapy should be initiated as soon as a patient's clinical condition allows. Patients with stage III colon cancer may benefit from adjuvant chemotherapy initiated within 3 weeks of surgery.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Protective effect of Korean red ginseng on oxaliplatin-mediated splenomegaly in colon cancer
Jeonghyun Kang, Joon Seong Park, Sung Gwe Ahn, Jin Hong Lim, Seung Hyuk Baik, Dong Sup Yoon, Kang Young Lee, Joon Jeong
Ann Surg Treat Res. 2018;95(3):161-167. doi: 10.4174/astr.2018.95.3.161.Weighing the benefits of lymphadenectomy in early-stage colorectal cancer
Seung Min Baik, Ryung-Ah Lee
Ann Surg Treat Res. 2023;105(5):245-251. doi: 10.4174/astr.2023.105.5.245.
Reference
-
1. Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990; 322:352–358.2. Wolmark N, Rockette H, Fisher B, Wickerham DL, Redmond C, Fisher ER, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol. 1993; 11:1879–1887.3. Bleiberg H. Adjuvant therapy in highrisk colon cancer. Semin Oncol. 2000; 27:5 Suppl 10. 48–59.4. Hershman D, Hall MJ, Wang X, Jacobson JS, McBride R, Grann VR, et al. Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer. 2006; 107:2581–2588.5. Cree M, Tonita J, Turner D, Nugent Z, Alvi R, Barss R, et al. Comparison of treatment received versus long-standing guidelines for stage III colon and stage II/III rectal cancer patients diagnosed in Alberta, Saskatchewan, and Manitoba in 2004. Clin Colorectal Cancer. 2009; 8:141–145.6. Winget M, Hossain S, Yasui Y, Scarfe A. Characteristics of patients with stage III colon adenocarcinoma who fail to receive guideline-recommended treatment. Cancer. 2010; 116:4849–4856.7. Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001; 93:850–857.8. Jessup JM, Stewart A, Greene FL, Minsky BD. Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA. 2005; 294:2703–2711.9. Ayanian JZ, Zaslavsky AM, Fuchs CS, Guadagnoli E, Creech CM, Cress RD, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol. 2003; 21:1293–1300.10. Labianca R, Nordlinger B, Beretta GD, Brouquet A, Cervantes A. ESMO Guidelines Working Group. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant treatment and followup. Ann Oncol. 2010; 21:Suppl 5. v70–v77.11. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN Guidelines): Colon/rectal cancer. ver. 1. 2010 [Internet]. Fort Wathington: National Comprehensive Cancer Network;c2015. cited 2015 Feb 16. Available from: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.12. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer;2010.13. McCulloch P, Choy A. Effect of menstrual phase on surgical treatment of breast cancer. Lancet. 1994; 344:402–403.14. Fidler IJ, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994; 79:185–188.15. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990; 82:4–6.16. Gunduz N, Fisher B, Saffer EA. Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res. 1979; 39:3861–3865.17. Goldie JH, Coldman AJ. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep. 1979; 63:1727–1733.18. Arkenau HT, Bermann A, Rettig K, Strohmeyer G, Porschen R. Arbeitsgemeinschaft Gastrointestinale Onkologie. 5-Fluorouracil plus leucovorin is an effective adjuvant chemotherapy in curatively resected stage III colon cancer: long-term follow-up results of the adjCCA-01 trial. Ann Oncol. 2003; 14:395–399.19. Chau I, Norman AR, Cunningham D, Tait D, Ross PJ, Iveson T, et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol. 2005; 16:549–557.20. Glimelius B, Dahl O, Cedermark B, Jakobsen A, Bentzen SM, Starkhammar H, et al. Adjuvant chemotherapy in colorectal cancer: a joint analysis of randomised trials by the Nordic Gastrointestinal Tumour Adjuvant Therapy Group. Acta Oncol. 2005; 44:904–912.21. Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and metaanalysis. JAMA. 2011; 305:2335–2342.22. Munireddy S, Kavalukas SL, Barbul A. Intra-abdominal healing: gastrointestinal tract and adhesions. Surg Clin North Am. 2010; 90:1227–1236.23. van der Kolk BM, de Man BM, Wobbes T, Hendriks T. Is early post-operative treatment with 5-fluorouracil possible without affecting anastomotic strength in the intestine? Br J Cancer. 1999; 79:545–550.24. Konstantinidis HD, Slavakis AP, Ballas KD, Sioga AC, Economou LD, Demertzidis CI, et al. The effect of capecitabine on the healing of colonic anastomoses in rats. Dis Colon Rectum. 2007; 50:89–96.25. Graf W, Weiber S, Glimelius B, Jiborn H, Påhlman L, Zederfeldt B. Influence of 5-fluorouracil and folinic acid on colonic healing: an experimental study in the rat. Br J Surg. 1992; 79:825–828.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparing the initiation of adjuvant chemotherapy after robotic and laparoscopic colon cancer surgeries: A case-controlled study with propensity score matching
- Adjuvant chemotherapy for patients with stage II high-risk and III colon cancer: Hindering factors to adherence and impact on long-term survival
- Oncologic Outcomes of Stage IIIA Colon Cancer for Different Chemotherapeutic Regimens
- Adjuvant Chemotherapy in Colon Cancer
- Chemotherapy for Colorecal Cancer