Ann Surg Treat Res.
2020 Aug;99(2):90-96. 10.4174/astr.2020.99.2.90.
Computed tomography based cross-sectional anatomy of the pelvis predicts surgical outcome after rectal cancer surgery
- Affiliations
-
- 1Department of Surgery, Ewha Womans University College of Medicine, Seoul, Korea
- KMID: 2504821
- DOI: http://doi.org/10.4174/astr.2020.99.2.90
Abstract
- Purpose
Narrow pelvis has been considered an adverse factor for postoperative and oncologic outcomes after rectal cancer surgery. The aim of this study was to investigate the validity of using only axial CT scan images to calculate the pelvic cross-sectional area for the prediction of adverse outcomes after rectal cancer surgery.
Methods
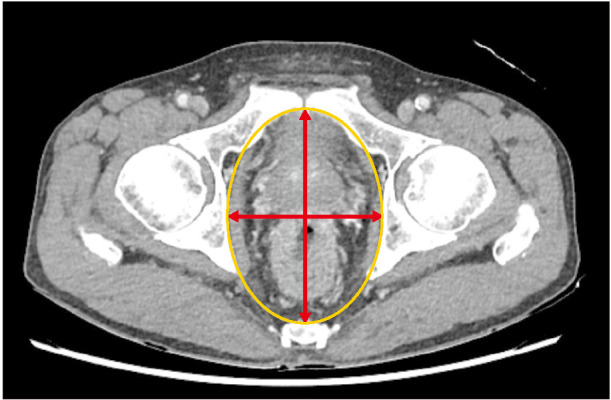
The medical records of patients who underwent rectal cancer surgery were reviewed and analyzed retrospectively. Axial images of CT scan were used to measure the pelvic cross-sectional area. Pelvic surgical site infection (SSI), positive resection margin, and early local recurrence were adopted as end-points to analyze the impact of pelvimetry on surgical outcome.
Results
The mean pelvic cross-sectional area was 84.3 ± 10.9 cm2. Males had significantly smaller pelvic areas than females (P < 0.001). Comparing pelvic cross-sectional areas according to the surgical outcomes, the results indicated that patients with pelvic SSI and local failure (positive resection margin or local recurrence within 1 year) have significantly smaller cross-sectional-area than SSI and local failure-free patients (P = 0.013 and P = 0.031). A calculated crosssectional area of 88.8 cm2 was determined as the cutoff value for the prediction of pelvic SSI and/or local failure, which was significant in a validating analysis.
Conclusion
The pelvic cross-sectional area obtained from a routine axial CT scan image was associated with pelvic SSI, positive resection margin, and early local recurrence. It might be an intuitive, feasible, and easily adoptable method for predicting surgical outcomes.
Keyword
Figure
Reference
-
1. Kim JS, Cho SY, Min BS, Kim NK. Risk factors for anastomotic leakage after laparoscopic intracorporeal colorectal anastomosis with a double stapling technique. J Am Coll Surg. 2009; 209:694–701. PMID: 19959036.2. Lenhard MS, Johnson TR, Weckbach S, Nikolaou K, Friese K, Hasbargen U. Pelvimetry revisited: analyzing cephalopelvic disproportion. Eur J Radiol. 2010; 74:e107–e111. PMID: 19443160.3. Neill MG, Lockwood GA, McCluskey SA, Fleshner NE. Preoperative evaluation of the “hostile pelvis” in radical prostatectomy with computed tomographic pelvimetry. BJU Int. 2007; 99:534–538. PMID: 17155982.4. Sporri S, Hanggi W, Braghetti A, Vock P, Schneider H. Pelvimetry by magnetic resonance imaging as a diagnostic tool to evaluate dystocia. Obstet Gynecol. 1997; 89:902–908. PMID: 9170462.5. Targarona EM, Balague C, Pernas JC, Martinez C, Berindoague R, Gich I, et al. Can we predict immediate outcome after laparoscopic rectal surgery? Multivariate analysis of clinical, anatomic, and pathologic features after 3-dimensional reconstruction of the pelvic anatomy. Ann Surg. 2008; 247:642–649. PMID: 18362627.6. Zaretsky MV, Alexander JM, McIntire DD, Hatab MR, Twickler DM, Leveno KJ. Magnetic resonance imaging pelvimetry and the prediction of labor dystocia. Obstet Gynecol. 2005; 106:919–926. PMID: 16260507.7. Sohn DK, Park SC, Kim MJ, Chang HJ, Han KS, Oh JH. Feasibility of transanal total mesorectal excision in cases with challenging patient and tumor characteristics. Ann Surg Treat Res. 2019; 96:123–130. PMID: 30838184.8. Zur Hausen G, Grone J, Kaufmann D, Niehues SM, Aschenbrenner K, Stroux A, et al. Influence of pelvic volume on surgical outcome after low anterior resection for rectal cancer. Int J Colorectal Dis. 2017; 32:1125–1135. PMID: 28315018.9. Baik SH, Kim NK, Lee KY, Sohn SK, Cho CH, Kim MJ, et al. Factors influencing pathologic results after total mesorectal excision for rectal cancer: analysis of consecutive 100 cases. Ann Surg Oncol. 2008; 15:721–728. PMID: 18058183.10. Boyle KM, Petty D, Chalmers AG, Quirke P, Cairns A, Finan PJ, et al. MRI assessment of the bony pelvis may help predict resectability of rectal cancer. Colorectal Dis. 2005; 7:232–240. PMID: 15859960.11. Wang C, Xiao Y, Qiu H, Yao J, Pan W. Factors affecting operating time in laparoscopic anterior resection of rectal cancer. World J Surg Oncol. 2014; 12:44. PMID: 24568575.12. Bertani E, Chiappa A, Della Vigna P, Radice D, Papis D, Cossu L, et al. The impact of pelvimetry on anastomotic leakage in a consecutive series of open, laparoscopic and robotic low anterior resections with total mesorectal excision for rectal cancer. Hepatogastroenterology. 2014; 61:1574–1581. PMID: 25436345.13. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. New York: Springer;2017.14. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011; 12:77. PMID: 21414208.15. Akiyoshi T, Kuroyanagi H, Oya M, Konishi T, Fukuda M, Fujimoto Y, et al. Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery. 2009; 146:483–489. PMID: 19715805.16. Keller TM, Rake A, Michel SC, Seifert B, Efe G, Treiber K, et al. Obstetric MR pelvimetry: reference values and evaluation of inter- and intraobserver error and intraindividual variability. Radiology. 2003; 227:37–43. PMID: 12601187.17. Korhonen U, Solja R, Laitinen J, Heinonen S, Taipale P. MR pelvimetry measurements, analysis of inter- and intra-observer variation. Eur J Radiol. 2010; 75:e56–e61. PMID: 20006454.18. Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, et al. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg. 2013; 257:665–671. PMID: 23333881.19. Yamamoto S, Fujita S, Akasu T, Inada R, Moriya Y, Yamamoto S. Risk factors for anastomotic leakage after laparoscopic surgery for rectal cancer using a stapling technique. Surg Laparosc Endosc Percutan Tech. 2012; 22:239–243. PMID: 22678320.20. Choi DH, Hwang JK, Ko YT, Jang HJ, Shin HK, Lee YC, et al. Risk factors for anastomotic leakage after laparoscopic rectal resection. J Korean Soc Coloproctol. 2010; 26:265–273. PMID: 21152228.21. Huh JW, Kim HR, Kim YJ. Anastomotic leakage after laparoscopic resection of rectal cancer: the impact of fibrin glue. Am J Surg. 2010; 199:435–441. PMID: 19481197.22. Joh YG, Kim SH, Hahn KY, Stulberg J, Chung CS, Lee DK. Anastomotic leakage after laparoscopic protectomy can be managed by a minimally invasive approach. Dis Colon Rectum. 2009; 52:91–96. PMID: 19273962.23. Kang J, Lee HB, Cha JH, Hur H, Min BS, Baik SH, et al. Feasibility and impact on surgical outcomes of modified double-stapling technique for patients undergoing laparoscopic anterior resection. J Gastrointest Surg. 2013; 17:771–775. PMID: 23288715.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anatomic Basis of Sharp Pelvic Dissection for Curative Resection of Rectal Cancer
- Pelvic Exenteration: Surgical Approaches
- Leonardo da Vinci, a Pioneer of the Sectional Anatomy
- Sex Disparities in Rectal Cancer Surgery: An In-Depth Analysis of Surgical Approaches and Outcomes
- Improved Sectioned Images of the Female Pelvis Showing Detailed Urogenital and Neighboring Structures