Intest Res.
2020 Jul;18(3):315-324. 10.5217/ir.2019.09179.
Factors associated with the survival of colorectal cancer in Mexico
- Affiliations
-
- 1Institute of Public Health, Veracruzana University, Veracruz, Mexico
- 2State Cancer Center, Secretary of Health of the State of Veracruz, Veracruz, Mexico
- KMID: 2504587
- DOI: http://doi.org/10.5217/ir.2019.09179
Abstract
- Background/Aims
Colorectal cancer (CRC) is a public health problem. In Mexico, there have been no recent studies conducted on survival in terms of this pathology or on the influence of prognostic factors. The study aims to determine the probability of survival in patients with CRC presence of low levels of schooling and a rural population, adjusted for clinical stage and type of treatment.
Methods
A retrospective study was conducted in a cohort of 305 patients with CRC treated at State Cancer Center, located in Veracruz-Mexico; the follow-up period of 60 months (2012–2016). The survival probability was calculated using the Kaplan-Meier estimator and the log-rank test with 95% confidence intervals (CIs). Prognostic factors were determined using hazard ratio (HR) multivariate Cox regression analysis.
Results
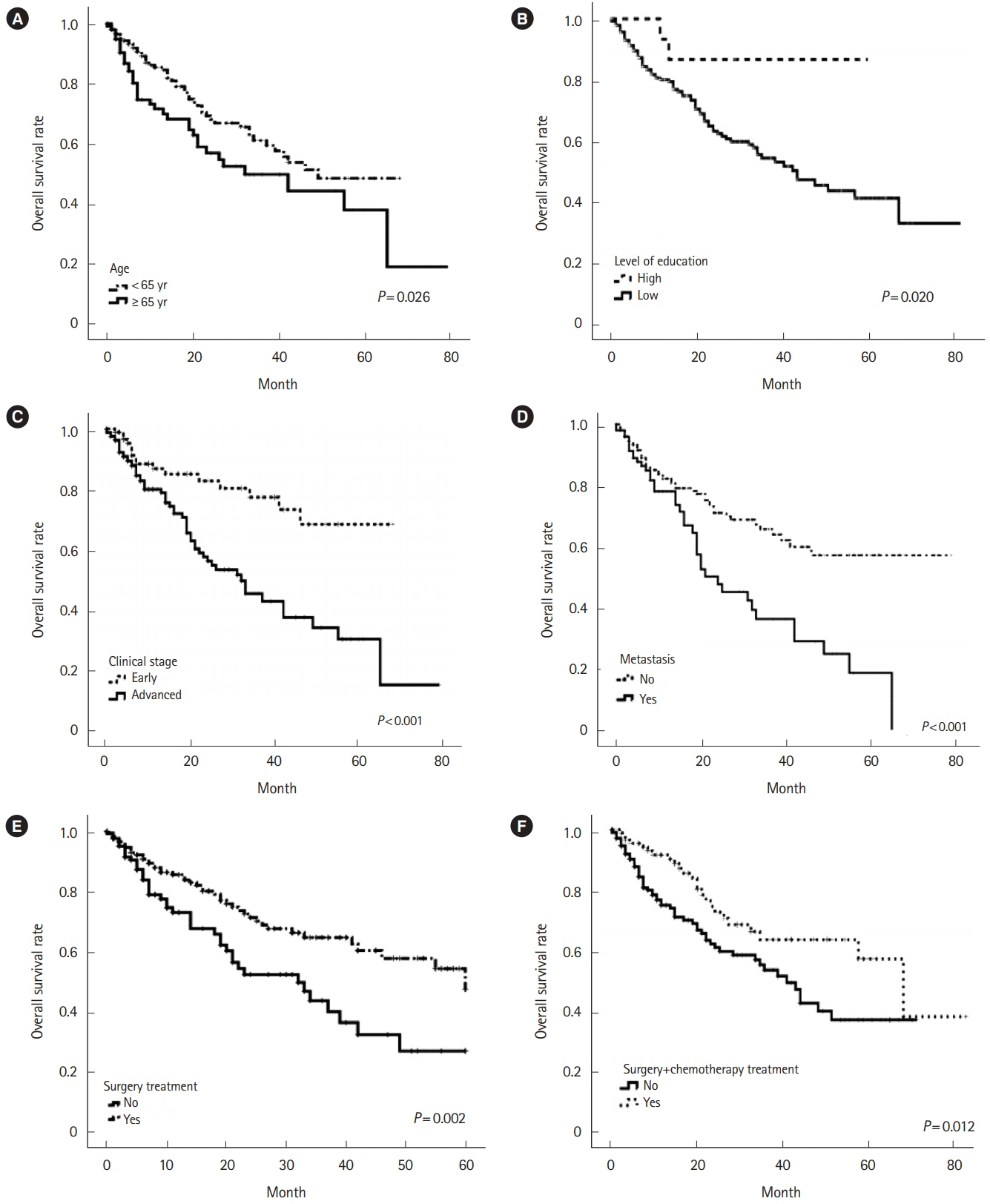
Overall survival was 40% at 60 months. Subjects in the age group ≥ 65 years had a low survival rate of 28% (P= 0.026) and an advanced clinical stage of 22% (P< 0.001). Of the patients with bone metastasis, none survived longer than 5 years (P= 0.008). With respect to the unfavorable prognostic factors identified in the multivariate analysis, a decreased level of schooling was associated with an HR of 7.6 (95% CI, 1.1–54.7), advanced clinical stage was associated with an HR of 2.1 (95% CI, 1.2–4.0), and the presence of metastasis had an HR of 1.8 (95% CI, 1.1–2.9).
Conclusions
Poor prognostic factors include an advanced clinical stage, the presence of metastasis and a low level of schooling. These findings confirm the importance of screening for early diagnosis, diminishing the barriers to accessing treatment and prospectively monitoring the population.
Figure
Cited by 1 articles
-
Education levels and survival in colorectal cancer: is there really an obvious association?
Bruna Valiati, Rodrigo Oliva Perez, Paulo Gustavo Kotze
Intest Res. 2020;18(3):247-248. doi: 10.5217/ir.2020.00064.
Reference
-
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017; 67:177–193.
Article3. Estadísticas a propósito del… día mundial contra el cancer: datos nacionales. National Institute of Statistics and Geography Web site. https://www.inegi.org.mx/contenidos/saladeprensa/aproposito/2018/cancer2018_nal.pdf. Updated February 2, 2018. Accessed March 5, 2020.4. Le H, Ziogas A, Lipkin SM, Zell JA. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol Biomarkers Prev. 2008; 17:1950–1962.
Article5. Marmot M. Social justice, epidemiology and health inequalities. Eur J Epidemiol. 2017; 32:537–546.
Article6. Fleming ST, Mackley HB, Camacho F, et al. Clinical, sociodemographic, and service provider determinants of guideline concordant colorectal cancer care for Appalachian residents. J Rural Health. 2014; 30:27–39.
Article7. Lai Y, Wang C, Civan JM, et al. Effects of cancer stage and treatment differences on racial disparities in survival from colon cancer: a United States population-based study. Gastroenterology. 2016; 150:1135–1146.
Article8. Beckmann KR, Bennett A, Young GP, et al. Sociodemographic disparities in survival from colorectal cancer in South Australia: a population-wide data linkage study. BMC Health Serv Res. 2016; 16:24.
Article9. Parreira VG, Meira KC, Guimarães RM. Socioeconomic differentials and mortality from colorectal cancer in large cities in Brazil. Ecancermedicalscience. 2016; 10:614.10. Buie WD, Attard JA. Follow-up recommendations for colon cancer. Clin Colon Rectal Surg. 2005; 18:232–243.
Article11. Van Cutsem E, Borràs JM, Castells A, et al. Improving outcomes in colorectal cancer: where do we go from here? Eur J Cancer. 2013; 49:2476–2485.
Article12. Loupakis F, Yang D, Yau L, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015; 107–dju427.
Article13. Pruitt SL, Harzke AJ, Davidson NO, Schootman M. Do diagnostic and treatment delays for colorectal cancer increase risk of death? Cancer Causes Control. 2013; 24:961–977.
Article14. Hurwitz HI, Tebbutt NC, Kabbinavar F, et al. Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials. Oncologist. 2013; 18:1004–1012.
Article15. Ciani O, Buyse M, Garside R, et al. Meta-analyses of randomized controlled trials show suboptimal validity of surrogate outcomes for overall survival in advanced colorectal cancer. J Clin Epidemiol. 2015; 68:833–842.
Article16. Neugut AI, Matasar M, Wang X, et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J Clin Oncol. 2006; 24:2368–2375.
Article17. Ling CR, Wang R, Wang MJ, Ping J, Zhuang W. Prognosis and value of preoperative radiotherapy in locally advanced rectal signet-ring cell carcinoma. Sci Rep. 2017; 7:45334.
Article18. Lejeune C, Sassi F, Ellis L, et al. Socio-economic disparities in access to treatment and their impact on colorectal cancer survival. Int J Epidemiol. 2010; 39:710–717.
Article19. Mondaca S, Villalón C, Leal JL, et al. Benefit of adjuvant 5-fluorouracil based chemotherapy for colon cancer: a retrospective cohort study. Rev Med Chil. 2016; 144:145–151.20. Armando C, Bravo LE, Clín P, García LS, Collazos P. Colorectal cancer incidence, mortality and survival in Cali, Colombia, 1962-2012. Salud Publica Mex. 2014; 56:457–464.21. Leufkens AM, Van Duijnhoven FJ, Boshuizen HC, et al. Educational level and risk of colorectal cancer in EPIC with specific reference to tumor location. Int J Cancer. 2012; 130:622–630.
Article22. Cavalli-Björkman N, Lambe M, Eaker S, Sandin F, Glimelius B. Differences according to educational level in the management and survival of colorectal cancer in Sweden. Eur J Cancer. 2011; 47:1398–1406.
Article23. Rasouli MA, Moradi G, Roshani D, Nikkhoo B, Ghaderi E, Ghaytasi B. Prognostic factors and survival of colorectal cancer in Kurdistan province, Iran: a population-based study (2009-2014). Medicine (Baltimore). 2017; 96:e5941.24. Sonnenberg A, Turner KO, Genta RM. Ethnic variations in the occurrence of colonic neoplasms. United European Gastroenterol J. 2017; 5:424–431.
Article25. Kim TJ, Kim ER, Hong SN, Chang DK, Kim YH. Long-term outcome and prognostic factors of sporadic colorectal cancer in young patients: a large institutional-based retrospective study. Medicine (Baltimore). 2016; 95:e3641.26. Robbins AS, Siegel RL, Jemal A. Racial disparities in stage-specific colorectal cancer mortality rates from 1985 to 2008. J Clin Oncol. 2012; 30:401–405.
Article27. Sharkas GF, Arqoub KH, Khader YS, et al. Colorectal cancer in Jordan: survival rate and its related factors. J Oncol. 2017; 2017:3180762.
Article28. White A, Joseph D, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001-2009): findings from the CONCORD-2 study. Cancer. 2017; 123 Suppl 24(Suppl 24):5014–5036.
Article29. Ryuk JP, Choi GS, Park JS, et al. Predictive factors and the prognosis of recurrence of colorectal cancer within 2 years after curative resection. Ann Surg Treat Res. 2014; 86:143–151.
Article30. Neuman HB, O’Connor ES, Weiss J, et al. Surgical treatment of colon cancer in patients aged 80 years and older: analysis of 31,574 patients in the SEER-Medicare database. Cancer. 2013; 119:639–647.
Article31. Liu F, Zhao J, Xie J, et al. Prognostic risk factors in patients with bone metastasis from colorectal cancer. Tumour Biol. 2016; 37:16127–16134.
Article32. Hsieh MC, Thompson T, Wu XC, et al. The effect of comorbidity on the use of adjuvant chemotherapy and type of regimen for curatively resected stage III colon cancer patients. Cancer Med. 2016; 5:871–880.
Article33. Tashiro J, Yamaguchi S, Ishii T, et al. Inferior oncological prognosis of surgery without oral chemotherapy for stage III colon cancer in clinical settings. World J Surg Oncol. 2014; 12:145.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Calcium, Vitamin D, and Colorectal Cancer
- Prognostic Value of HSP 70 in Colorectal Cancer
- Reply on "Data on the Characteristics and the Survival of Korean Patients With Colorectal Cancer From the Korea Central Cancer Registry"
- Commentary on "Data on the Characteristics and the Survival of Korean Patients With Colorectal Cancer From the Korea Central Cancer Registry"
- Current Status of Chemotherapy in Colorectal Cancer: Updated Treatment Strategies