Intest Res.
2020 Jul;18(3):282-288. 10.5217/ir.2019.09140.
Safety of tumor necrosis factor inhibitor use in patients with concomitant malignancy
- Affiliations
-
- 1Department of Medicine, VA North Texas Healthcare System, Dallas, TX, USA
- 2Department of Medicine, University of Texas Southwestern Medical Center, Dallas, TX, USA
- 3Department of Pharmacy, VA North Texas Healthcare System, Dallas, TX, USA
- 4College of Nursing and Health Innovation, University of Texas at Arlington, Arlington, TX, USA
- 5Division of Gastroenterology, The University of Texas at Austin Dell Medical School, Austin, TX, USA
- KMID: 2504583
- DOI: http://doi.org/10.5217/ir.2019.09140
Abstract
- Background/Aims
Safety for tumor necrosis factor inhibitors (TNFi) in cancer has been focused on risk of incident malignancies, but studies on prognostic effects have been scarce. We determined survival and recurrence rates at 1, 2, and 5 years after cancer diagnosis in patients with and without concurrent TNFi use.
Methods
Chart reviews were performed between 1996 and 2015 at the VA North Texas Healthcare System. Cases were patients with inflammatory disease, concomitant malignancy, and TNFi use while controls were patients with inflammatory disease, concomitant malignancy but no TNFi use. Cases and controls were matched for type of malignancy. Analysis was performed with log-rank tests on Kaplan-Meier curves.
Results
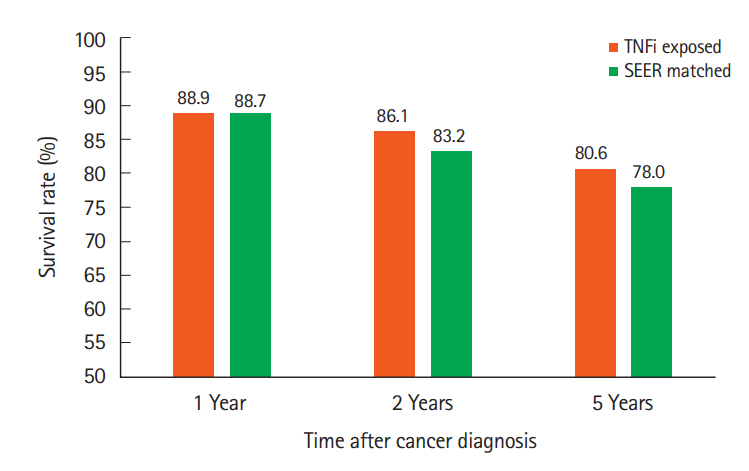
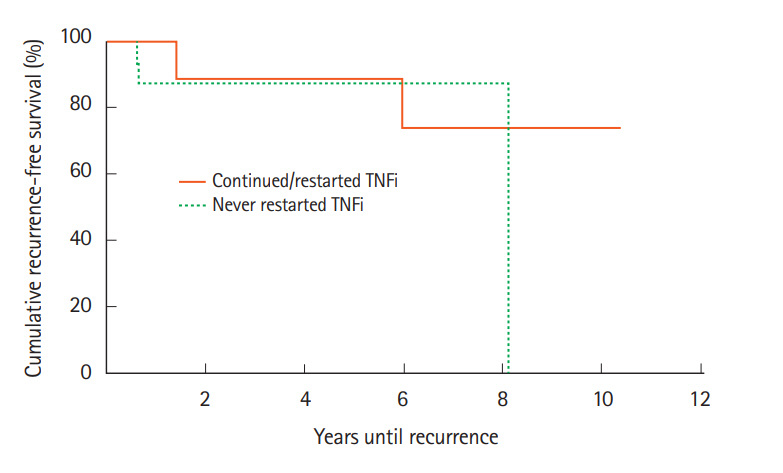
Thirty-six cases and 72 controls were identified. For cases, survival at 1, 2, and 5 years were 32 (89%), 31 (86%), and 29 (81%) compared to 63 (90%), 61 (87%), and 51 (73%) for the control group (P=0.985). For cases, recurrence rates at 1, 2, and 5 years were 3 (8%), 5 (14%), and 6 (17%) compared to 2 (3%), 5 (7%), and 7 (10%) for the control group (P=0.158).
Conclusions
Our findings suggest TNFi may be safely used in select inflammatory disease patients with concurrent cancer if therapy is needed for proper disease control. However, case-by-case consideration in conjunction with an oncologist is recommended while considering the apparent safety of TNFi for patients suffering from active inflammatory diseases despite having a concomitant malignancy.
Keyword
Figure
Cited by 2 articles
-
Use of Disease-modifying Antirheumatic Drugs After Cancer Diagnosis in Rheumatoid Arthritis Patients
Young Bin Joo, Seung Min Jeong, Yune-Jung Park, Ki-Jo Kim, Kyung-Su Park
J Rheum Dis. 2022;29(3):162-170. doi: 10.4078/jrd.2022.29.3.162.Tumor necrosis factor-α inhibitor use in patients with malignancy: is it safe?
Gyu Man Oh, Won Moon
Intest Res. 2020;18(3):245-246. doi: 10.5217/ir.2020.00061.
Reference
-
1. Elandt K, Aletaha D. Treating rheumatic patients with a malignancy. Arthritis Res Ther. 2011; 13:223.
Article2. Nguyen GC. First do no harm: is it safe to use immunosuppressants in inflammatory bowel disease patients with prior cancer? Gastroenterology. 2016; 151:22–24.
Article3. Penn I. Evaluation of transplant candidates with pre-existing malignancies. Ann Transplant. 1997; 2:14–17.4. Center for Drug Evaluation and Research. Drug Safety Information for Heathcare Professionals - Questions and Answers - TNF Blockers 8/25/2009. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-tumor-necrosis-factor-tnf-blockers-marketed-remicade-enbrel-humira-cimzia-and-simponi.5. Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006; 295:2275–2285.
Article6. Mamtani R, Clark AS, Scott FI, et al. Association between breast cancer recurrence and immunosuppression in rheumatoid arthritis and inflammatory bowel disease: a cohort study. Arthritis Rheumatol. 2016; 68:2403–2411.
Article7. Micic D, Komaki Y, Alavanja A, Rubin DT, Sakuraba A. Risk of cancer recurrence among individuals exposed to antitumor necrosis factor therapy: a systematic review and meta-analysis of observational studies. J Clin Gastroenterol. 2019; 53:e1–e11.8. Raaschou P, Simard JF, Neovius M, Askling J; Anti-Rheumatic Therapy in Sweden Study Group. Does cancer that occurs during or after anti-tumor necrosis factor therapy have a worse prognosis? A national assessment of overall and site-specific cancer survival in rheumatoid arthritis patients treated with biologic agents. Arthritis Rheum. 2011; 63:1812–1822.
Article9. Silva-Fernández L, Lunt M, Kearsley-Fleet L, et al. The incidence of cancer in patients with rheumatoid arthritis and a prior malignancy who receive TNF inhibitors or rituximab: results from the British Society for Rheumatology Biologics Register-Rheumatoid Arthritis. Rheumatology (Oxford). 2016; 55:2033–2039.
Article10. Poullenot F, Seksik P, Beaugerie L, et al. Risk of incident cancer in inflammatory bowel disease patients starting anti-TNF therapy while having recent malignancy. Inflamm Bowel Dis. 2016; 22:1362–1369.
Article11. Axelrad J, Bernheim O, Colombel JF, et al. Risk of new or recurrent cancer in patients with inflammatory bowel disease and previous cancer exposed to immunosuppressive and anti-tumor necrosis factor agents. Clin Gastroenterol Hepatol. 2016; 14:58–64.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tumor necrosis factor-α inhibitor use in patients with malignancy: is it safe?
- Patients treated with a tumor necrosis factor-alpha inhibitor are more likely to develop extrapulmonary tuberculosis
- Interstitial Lung Disease That Occurred during Treatment with Etanercept (Enbrel(R)) of Psoriasis Patients
- Effect of Immunomodulators and Biologic Agents on Malignancy in Patients with Inflammatory Bowel Disease
- Changes of Serum Tumor Necrosis Factor a and Interleukin 1B in the Sepsis of Neonates