Highly prevalent BRAF V600E and low-frequency TERT promoter mutations underlie papillary thyroid carcinoma in Koreans
- Affiliations
-
- 1Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Molecular Pathology Unit, Pathology Laboratory, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Cancer Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2504561
- DOI: http://doi.org/10.4132/jptm.2020.05.12
Abstract
- Background
The presence of telomerase reverse transcriptase (TERT) promoter mutations have been associated with a poor prognosis in patients with papillary thyroid carcinomas (PTC). The frequency of TERT promoter mutations varies widely depending on the population and the nature of the study.
Methods
Data were prospectively collected in 724 consecutive patients who underwent thyroidectomy for PTC from 2018 to 2019. Molecular testing for BRAF V600E and TERT promoter mutations was performed in all cases.
Results
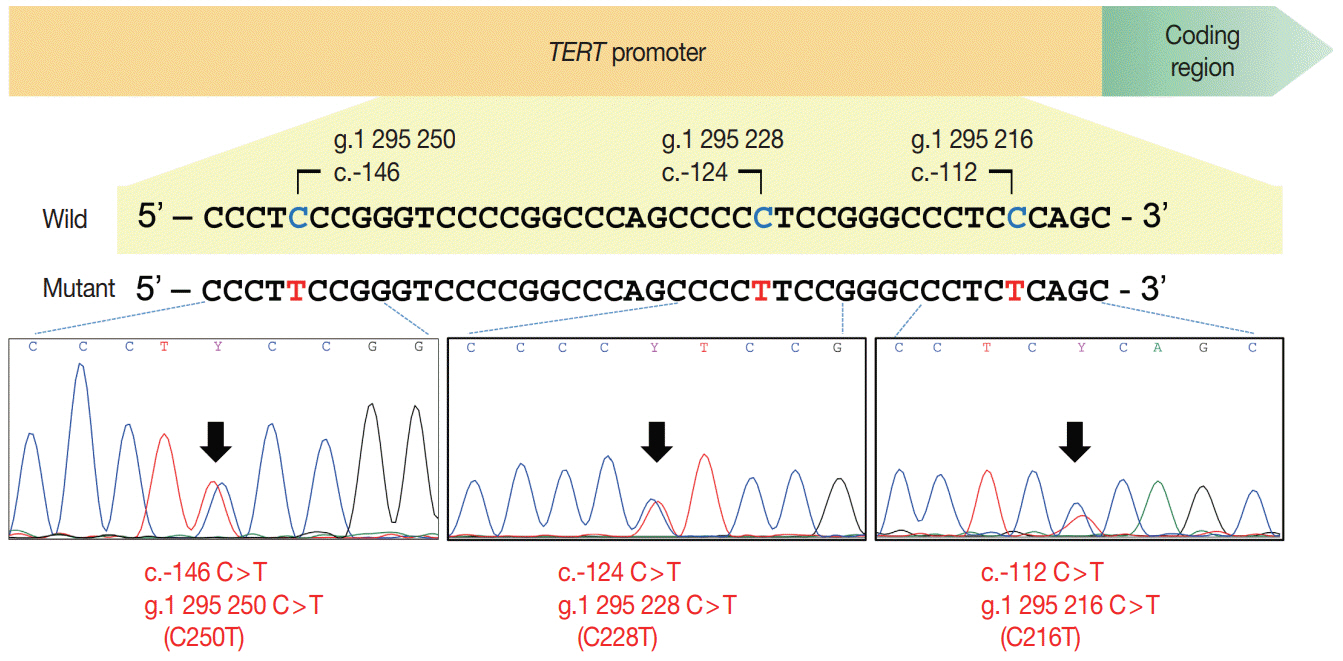
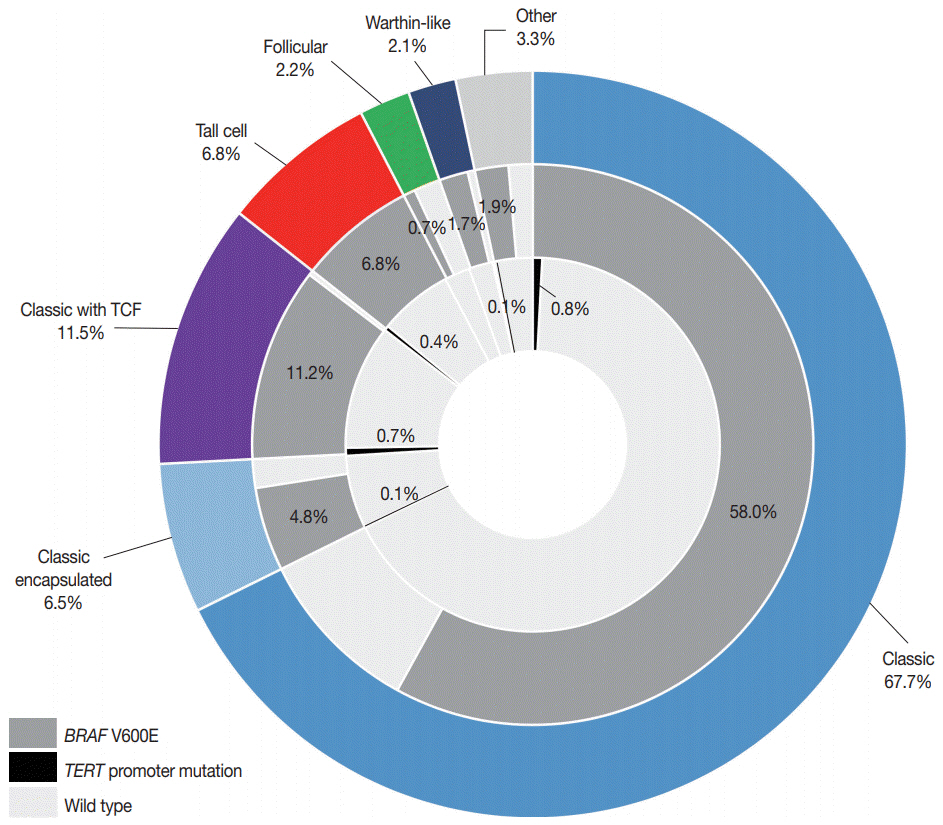
TERT promoter alterations in two hotspots (C228T and C250T) and C216T were found in 16 (2.2%) and 4 (0.6%) of all PTCs, respectively. The hotspot mutations were significantly associated with older age at diagnosis, larger tumor size, extrathyroidal extension, higher pathologic T category, lateral lymph node metastasis, and higher American Thyroid Association recurrence risk. The patients with C216T variant were younger and had a lower American Thyroid Association recurrence risk than those with hotspot mutations. Concurrent BRAF V600E was found in 19 of 20 cases with TERT promoter mutations. Of 518 microcarcinomas measuring ≤1.0 cm in size, hotspot mutations and C216T variants were detected in five (1.0%) and three (0.6%) cases, respectively.
Conclusions
Our study indicates a low frequency of TERT promoter mutations in Korean patients with PTC and supports previous findings that TERT promoter mutations are more common in older patients with unfavorable clinicopathologic features and BRAF V600E. TERT promoter mutations in patients with microcarcinoma are uncommon and may have a limited role in risk stratification. The C216T variant seems to have no clinicopathologic effect on PTC.
Figure
Cited by 6 articles
-
Clinicopathological Characteristics and Recurrence-Free Survival of Rare Variants of Papillary Thyroid Carcinomas in Korea: A Retrospective Study
Mijin Kim, Sun Wook Cho, Young Joo Park, Hwa Young Ahn, Hee Sung Kim, Yong Joon Suh, Dughyun Choi, Bu Kyung Kim, Go Eun Yang, Il-Seok Park, Ka Hee Yi, Chan Kwon Jung, Bo Hyun Kim
Endocrinol Metab. 2021;36(3):619-627. doi: 10.3803/EnM.2021.974.Frequency of
TERT Promoter Mutations in Real-World Analysis of 2,092 Thyroid Carcinoma Patients (Endocrinol Metab 2022;37:652-63, Heera Yang et al.)
Sue Youn Kim, Chan Kwon Jung
Endocrinol Metab. 2022;37(6):947-948. doi: 10.3803/EnM.2022.1596.Frequency of
TERT Promoter Mutations in Real-World Analysis of 2,092 Thyroid Carcinoma Patients (Endocrinol Metab 2022;37:652-63, Heera Yang et al.)
Hyunju Park, Jae Hoon Chung
Endocrinol Metab. 2022;37(6):949-950. doi: 10.3803/EnM.2022.601.2023 Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules
Young Joo Park, Eun Kyung Lee, Young Shin Song, Soo Hwan Kang, Bon Seok Koo, Sun Wook Kim, Dong Gyu Na, Seung-Kuk Baek, So Won Oh, Min Kyoung Lee, Sang-Woo Lee, Young Ah Lee, Yong Sang Lee, Ji Ye Lee, Dong-Jun Lim, Leehi Joo, Yuh-Seog Jung, Chan Kwon Jung, Yoon Young Cho, Yun Jae Chung, Won Bae Kim, Ka Hee Yi, Ho-Cheol Kang, Do Joon Park
Int J Thyroidol. 2023;16(1):1-31. doi: 10.11106/ijt.2023.16.1.1.Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules 2024
Young Joo Park, Eun Kyung Lee, Young Shin Song, Su Hwan Kang, Bon Seok Koo, Sun Wook Kim, Dong Gyu Na, Seung-Kuk Baek, So Won Oh, Min Kyoung Lee, Sang-Woo Lee, Young Ah Lee, Yong Sang Lee, Ji Ye Lee, Dong-Jun Lim, Leehi Joo, Yuh-Seog Jung, Chan Kwon Jung, Yoon Young Cho, Yun Jae Chung, Won Bae Kim, Ka Hee Yi, Ho-Cheol Kang, Do Joon Park
Int J Thyroidol. 2024;17(1):208-244. doi: 10.11106/ijt.2024.17.1.208.Korean Thyroid Association Guidelines on the Management of Differentiated Thyroid Cancers; Part I. Initial Management of Differentiated Thyroid Cancers - Chapter 5. Evaluation of Recurrence Risk Postoperatively and Initial Risk Stratification in Differentiated Thyroid Cancer 2024
Eun Kyung Lee, Young Shin Song, Ho-Cheol Kang, Sun Wook Kim, Dong Gyu Na, Shin Je Moon, Dong-Jun Lim, Kyong Yeun Jung, Yun Jae Chung, Chan Kwon Jung, Young Joo Park
Int J Thyroidol. 2024;17(1):68-96. doi: 10.11106/ijt.2024.17.1.68.
Reference
-
References
1. Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016; 375:614–7.
Article2. Ahn HS, Kim HJ, Kim KH, et al. Thyroid cancer screening in South Korea increases detection of papillary cancers with no impact on other subtypes or thyroid cancer mortality. Thyroid. 2016; 26:1535–40.
Article3. Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer “epidemic”: screening and overdiagnosis. N Engl J Med. 2014; 371:1765–7.4. Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019; 51:417–30.
Article5. Park S, Oh CM, Cho H, et al. Association between screening and the thyroid cancer “epidemic” in South Korea: evidence from a nationwide study. BMJ. 2016; 355:i5745.
Article6. Jeon MJ, Kim WG, Kim TH, et al. Disease-specific mortality of differentiated thyroid cancer patients in Korea: a multicenter cohort study. Endocrinol Metab (Seoul). 2017; 32:434–41.
Article7. Lloyd RV, Osamura RY, Klöppel G, Rosai J. WHO classification of tumours of endocrine organs. 4th ed. Lyon: International Agency for Research on Cancer (IARC);2017. p. 65–91.8. Liu R, Bishop J, Zhu G, Zhang T, Ladenson PW, Xing M. Mortality risk stratification by combining BRAF V600E and TERT promoter mutations in papillary thyroid cancer: genetic duet of BRAF and TERT promoter mutations in thyroid cancer mortality. JAMA Oncol. 2017; 3:202–8.9. Kim TH, Kim YE, Ahn S, et al. TERT promoter mutations and long-term survival in patients with thyroid cancer. Endocr Relat Cancer. 2016; 23:813–23.10. Liu X, Qu S, Liu R, et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J Clin Endocrinol Metab. 2014; 99:E1130–6.11. Melo M, da Rocha AG, Vinagre J, et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2014; 99:E754–65.12. Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013; 339:959–61.13. Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013; 339:957–9.14. Bae JS, Kim Y, Jeon S, et al. Clinical utility of TERT promoter mutations and ALK rearrangement in thyroid cancer patients with a high prevalence of the BRAF V600E mutation. Diagn Pathol. 2016; 11:21.
Article15. Xing M, Liu R, Liu X, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014; 32:2718–26.16. Jung CK, Kim Y, Jeon S, Jo K, Lee S, Bae JS. Clinical utility of EZH1 mutations in the diagnosis of follicular-patterned thyroid tumors. Hum Pathol. 2018; 81:9–17.17. Landa I, Ganly I, Chan TA, et al. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab. 2013; 98:E1562–6.18. Liu X, Bishop J, Shan Y, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013; 20:603–10.19. Song YS, Lim JA, Choi H, et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer. 2016; 122:1370–9.20. Amin MB, Edge S, Greene F, et al. AJCC cancer staging manual. 8th ed. New York: Springer;2017. p. 873–90.21. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133.22. Jeong D, Jeong Y, Lee S, et al. Detection of BRAF(V600E) mutations in papillary thyroid carcinomas by peptide nucleic acid clamp real-time PCR: a comparison with direct sequencing. Korean J Pathol. 2012; 46:61–7.23. Yang J, Gong Y, Yan S, Chen H, Qin S, Gong R. Association between TERT promoter mutations and clinical behaviors in differentiated thyroid carcinoma: a systematic review and meta-analysis. Endocrine. 2020; 67:44–57.24. de Biase D, Gandolfi G, Ragazzi M, et al. TERT promoter mutations in papillary thyroid microcarcinomas. Thyroid. 2015; 25:1013–9.25. Yabuta T, Matsuse M, Hirokawa M, Yamashita S, Mitsutake N, Miyauchi A. TERT promoter mutations were not found in papillary thyroid microcarcinomas that showed disease progression on active surveillance. Thyroid. 2017; 27:1206–7.26. Ma X, Gong R, Wang R, et al. Recurrent TERT promoter mutations in non-small cell lung cancers. Lung Cancer. 2014; 86:369–73.27. Zhao Y, Gao Y, Chen Z, Hu X, Zhou F, He J. Low frequency of TERT promoter somatic mutation in 313 sporadic esophageal squamous cell carcinomas. Int J Cancer. 2014; 134:493–4.28. Song YS, Yoo SK, Kim HH, et al. Interaction of BRAF-induced ETS factors with mutant TERT promoter in papillary thyroid cancer. Endocr Relat Cancer. 2019; 26:629–41.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Low Prevalence of Somatic TERT Promoter Mutations in Classic Papillary Thyroid Carcinoma
- Mechanisms of TERT Reactivation and Its Interaction with BRAFV600E
- Thyroid Cancer, Iodine, and Gene Mutation
- Frequency of TERT Promoter Mutations in Real-World Analysis of 2,092 Thyroid Carcinoma Patients
- TERT Promoter Mutations and Tumor Persistence/Recurrence in Papillary Thyroid Cancer