J Pathol Transl Med.
2020 Jul;54(4):300-309. 10.4132/jptm.2020.06.08.
Analysis of PAX8 immunohistochemistry in lung cancers: a meta-analysis
- Affiliations
-
- 1Department of Thoracic and Cardiovascular Surgery, Chosun University Hospital, Chosun University School of Medicine, Gwangju, Korea
- 2Department of Internal Medicine, Daejeon Eulji University Hospital, Eulji University School of Medicine, Daejeon, Korea
- 3Department of Pathology, Daejeon Eulji University Hospital, Eulji University School of Medicine, Daejeon, Korea
- KMID: 2504560
- DOI: http://doi.org/10.4132/jptm.2020.06.08
Abstract
- Background
In this meta-analysis, we aimed to evaluate the PAX8 immunohistochemical expressions in primary lung cancers and metastatic cancers to the lung.
Methods
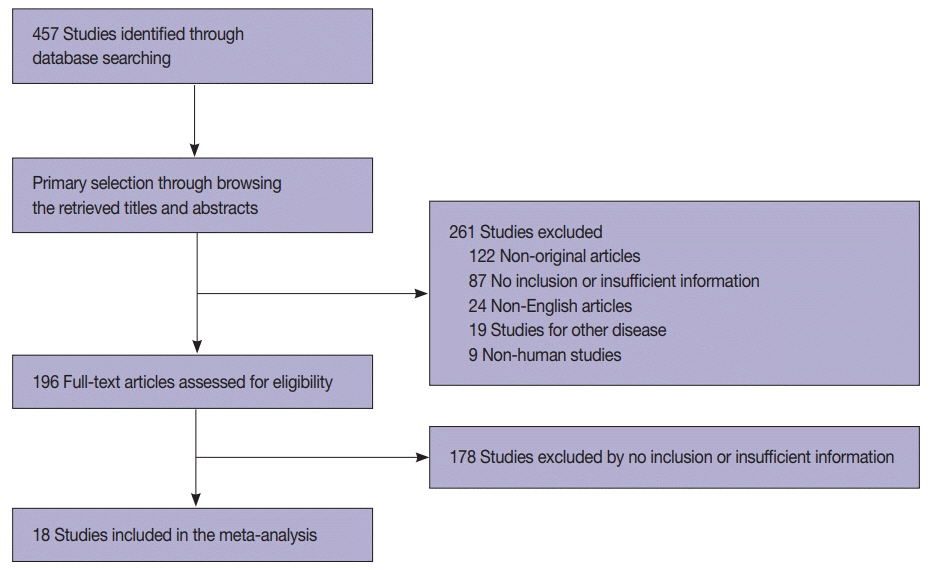
We identified and reviewed relevant articles from the PubMed databases. Ultimately, 18 articles were included in this meta-analysis. PAX8 expression rates were analyzed and compared between primary and metastatic lung cancers.
Results
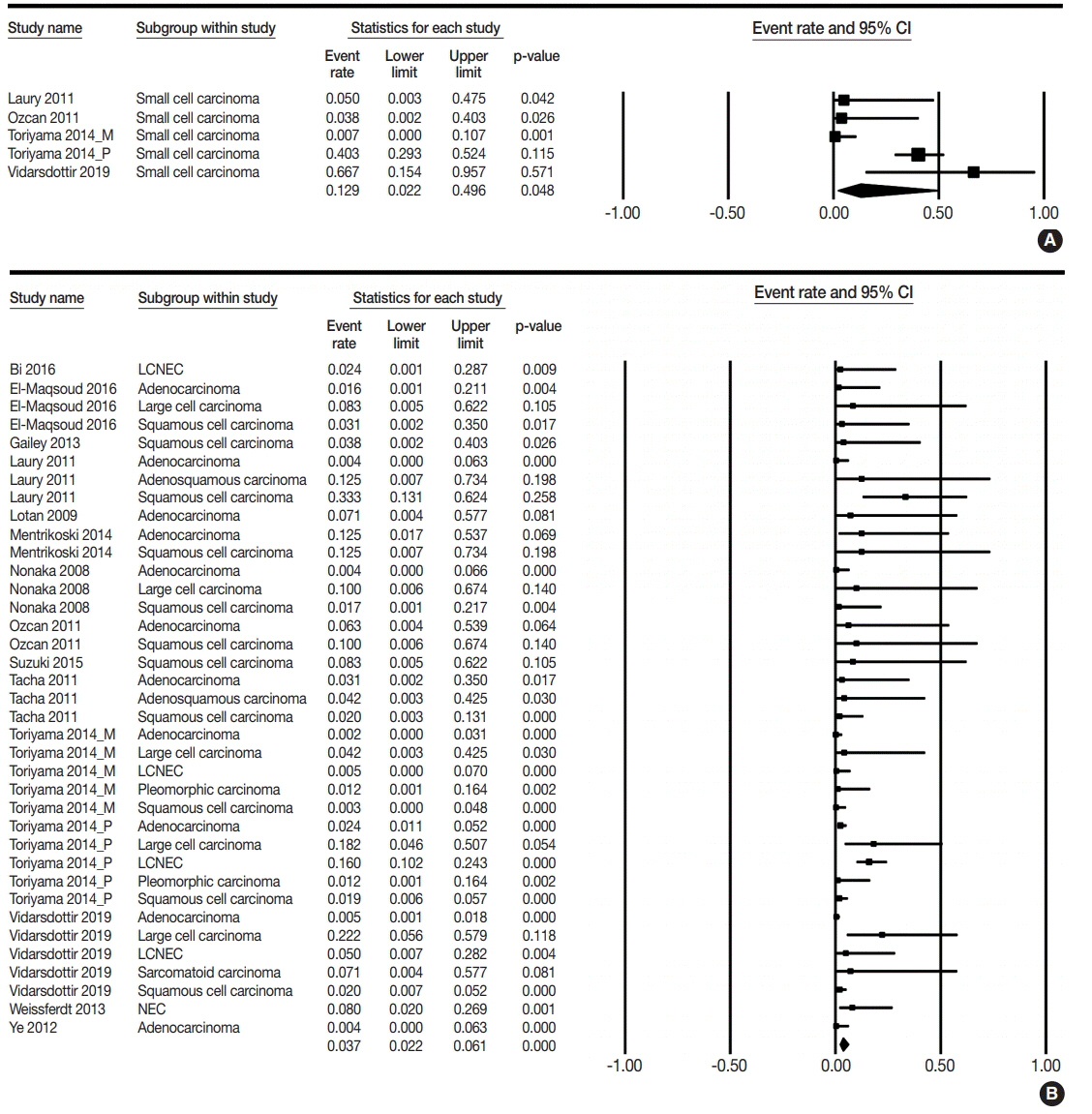
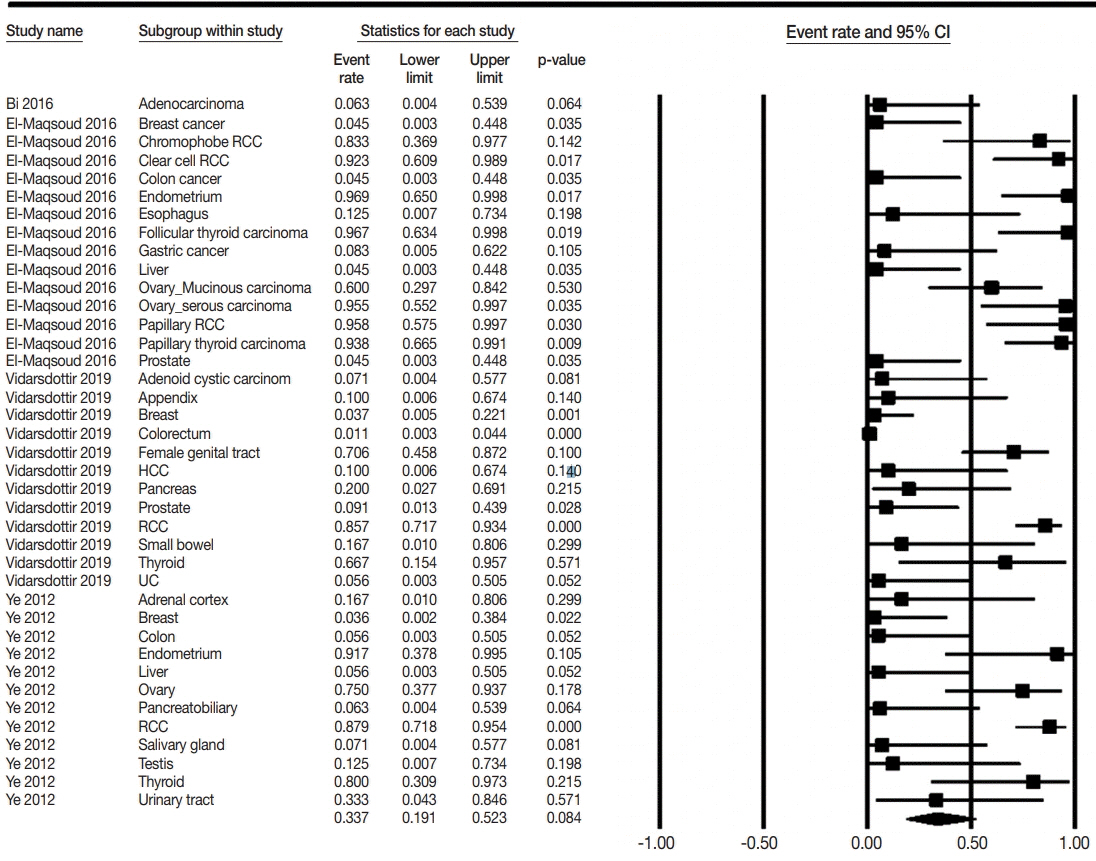
The PAX8 expression rate in primary lung cancers was 0.042 (95% confidence interval [CI], 0.025 to 0.071). PAX8 expression rates of small cell (0.129; 95% CI, 0.022 to 0.496) and non-small cell carcinomas of the lung (0.037; 95% CI, 0.022 to 0.061) were significantly different (p=.049 in a meta-regression test). However, the PAX8 expression rates of adenocarcinoma (0.013; 95% CI, 0.006 to 0.031) and squamous cell carcinoma (0.040; 95% CI, 0.016 to 0.097) were not significantly different. PAX8 expression rates of metastatic carcinomas to the lung varied, ranging from 1.8% to 94.9%. Metastatic carcinomas from the lung to other organs had a PAX8 expression rate of 6.3%. The PAX8 expression rates of metastatic carcinomas from the female genital organs, kidneys, and thyroid gland to the lung were higher than those of other metastatic carcinomas.
Conclusions
Primary lung cancers had a low PAX8 expression rate regardless of tumor subtype. However, the PAX8 expression rates of metastatic carcinomas from the female genital organs, kidneys, and thyroid were significantly higher than those of primary lung cancers.
Keyword
Figure
Reference
-
1. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart. 4th ed. Lyon: International Agency for Research on Cancer;2015.2. Brown AF, Sirohi D, Fukuoka J, et al. Tissue-preserving antibody cocktails to differentiate primary squamous cell carcinoma, adenocarcinoma, and small cell carcinoma of lung. Arch Pathol Lab Med. 2013; 137:1274–81.
Article3. Travis WD. Pathology of lung cancer. Clin Chest Med. 2002; 23:65–81.
Article4. Ye J, Hameed O, Findeis-Hosey JJ, et al. Diagnostic utility of PAX8, TTF-1 and napsin A for discriminating metastatic carcinoma from primary adenocarcinoma of the lung. Biotech Histochem. 2012; 87:30–4.
Article5. Bishop JA, Sharma R, Illei PB. Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Hum Pathol. 2010; 41:20–5.
Article6. Boggaram V. Thyroid transcription factor-1 (TTF-1/Nkx2.1/TITF1) gene regulation in the lung. Clin Sci (Lond). 2009; 116:27–35.
Article7. Ye J, Findeis-Hosey JJ, Yang Q, et al. Combination of napsin A and TTF-1 immunohistochemistry helps in differentiating primary lung adenocarcinoma from metastatic carcinoma in the lung. Appl Immunohistochem Mol Morphol. 2011; 19:313–7.
Article8. Asirvatham JR, Esposito MJ, Bhuiya TA. Role of PAX-8, CD5, and CD117 in distinguishing thymic carcinoma from poorly differentiated lung carcinoma. Appl Immunohistochem Mol Morphol. 2014; 22:372–6.
Article9. Bi R, Bai Q, Zhu X, et al. ALK rearrangement: a high-frequency alteration in ovarian metastasis from lung adenocarcinoma. Diagn Pathol. 2019; 14:96.
Article10. Bi Y, Deng Y, Li S, et al. Immunophenotypic and prognostic analysis of PAX8 and TTF-1 expressions in neuroendocrine carcinomas of thymic origin: A comparative study with their pulmonary counterparts. J Surg Oncol. 2016; 114:697–702.
Article11. El-Maqsoud NM, Tawfiek ER, Abdelmeged A, Rahman MF, Moustafa AA. The diagnostic utility of the triple markers Napsin A, TTF-1, and PAX8 in differentiating between primary and metastatic lung carcinomas. Tumour Biol. 2016; 37:3123–34.
Article12. Gailey MP, Bellizzi AM. Immunohistochemistry for the novel markers glypican 3, PAX8, and p40 (DeltaNp63) in squamous cell and urothelial carcinoma. Am J Clin Pathol. 2013; 140:872–80.13. Heidarpour M, Tavanafar Z. Diagnostic utility of PAX8 in differentiation of mullerian from non-mullerian tumors. Adv Biomed Res. 2014; 3:96.
Article14. Laury AR, Perets R, Piao H, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011; 35:816–26.
Article15. Lotan TL, Ye H, Melamed J, Wu XR, Shih Ie M, Epstein JI. Immunohistochemical panel to identify the primary site of invasive micropapillary carcinoma. Am J Surg Pathol. 2009; 33:1037–41.
Article16. Mentrikoski MJ, Wendroth SM, Wick MR. Immunohistochemical distinction of renal cell carcinoma from other carcinomas with clear-cell histomorphology: utility of CD10 and CA-125 in addition to PAX-2, PAX-8, RCCma, and adipophilin. Appl Immunohistochem Mol Morphol. 2014; 22:635–41.17. Nonaka D, Tang Y, Chiriboga L, Rivera M, Ghossein R. Diagnostic utility of thyroid transcription factors Pax8 and TTF-2 (FoxE1) in thyroid epithelial neoplasms. Mod Pathol. 2008; 21:192–200.
Article18. Ozcan A, Shen SS, Hamilton C, et al. PAX 8 expression in nonneoplastic tissues, primary tumors, and metastatic tumors: a comprehensive immunohistochemical study. Mod Pathol. 2011; 24:751–64.
Article19. Suzuki A, Hirokawa M, Takada N, et al. Diagnostic significance of PAX8 in thyroid squamous cell carcinoma. Endocr J. 2015; 62:991–5.
Article20. Tacha D, Qi W, Zhou D, Bremer R, Cheng L. PAX8 mouse monoclonal antibody [BC12] recognizes a restricted epitope and is highly sensitive in renal cell and ovarian cancers but does not cross-react with b cells and tumors of pancreatic origin. Appl Immunohistochem Mol Morphol. 2013; 21:59–63.
Article21. Tacha D, Zhou D, Cheng L. Expression of PAX8 in normal and neoplastic tissues: a comprehensive immunohistochemical study. Appl Immunohistochem Mol Morphol. 2011; 19:293–9.22. Toriyama A, Mori T, Sekine S, Yoshida A, Hino O, Tsuta K. Utility of PAX8 mouse monoclonal antibody in the diagnosis of thyroid, thymic, pleural and lung tumours: a comparison with polyclonal PAX8 antibody. Histopathology. 2014; 65:465–72.
Article23. Vidarsdottir H, Tran L, Nodin B, et al. Immunohistochemical profiles in primary lung cancers and epithelial pulmonary metastases. Hum Pathol. 2019; 84:221–30.
Article24. Weissferdt A, Tang X, Moran CA. Comparative immunohistochemical analysis of pulmonary and thymic neuroendocrine carcinomas using PAX8 and TTF-1. Mod Pathol. 2013; 26:1554–60.
Article25. Bowen NJ, Logani S, Dickerson EB, et al. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol. 2007; 104:331–7.
Article26. Kobel M, Kalloger SE, Boyd N, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 2008; 5:e232.
Article27. Nonaka D, Chiriboga L, Soslow RA. Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol. 2008; 32:1566–71.
Article28. Tong GX, Weeden EM, Hamele-Bena D, et al. Expression of PAX8 in nephrogenic adenoma and clear cell adenocarcinoma of the lower urinary tract: evidence of related histogenesis? Am J Surg Pathol. 2008; 32:1380–7.29. Tong GX, Yu WM, Beaubier NT, et al. Expression of PAX8 in normal and neoplastic renal tissues: an immunohistochemical study. Mod Pathol. 2009; 22:1218–27.
Article30. Albadine R, Schultz L, Illei P, et al. PAX8 (+)/p63 (-) immunostaining pattern in renal collecting duct carcinoma (CDC): a useful immunoprofile in the differential diagnosis of CDC versus urothelial carcinoma of upper urinary tract. Am J Surg Pathol. 2010; 34:965–9.31. Fabbro D, Di Loreto C, Beltrami CA, Belfiore A, Di Lauro R, Damante G. Expression of thyroid-specific transcription factors TTF-1 and PAX-8 in human thyroid neoplasms. Cancer Res. 1994; 54:4744–9.32. Fujiwara M, Taube J, Sharma M, McCalmont TH, Kim J. PAX8 discriminates ovarian metastases from adnexal tumors and other cutaneous metastases. J Cutan Pathol. 2010; 37:938–43.
Article33. Niu FY, Zhou Q, Yang JJ, et al. Distribution and prognosis of uncommon metastases from non-small cell lung cancer. BMC Cancer. 2016; 16:149.
Article34. Mazzaferri EL. Thyroid carcinoma: papillary and follicular. In : Mazzaferri EL, Samaan N, editors. Endocrine tumors. Cambridge: Blackwell Scientific Publications Inc;1993. p. 278–333.35. Lin JD, Weng HF, Ho YS. Clinical and pathological characteristics of secondary thyroid cancer. Thyroid. 1998; 8:149–53.
Article36. Nakhjavani MK, Gharib H, Goellner JR, van Heerden JA. Metastasis to the thyroid gland: a report of 43 cases. Cancer. 1997; 79:574–8.37. Kuhn E, Ragazzi M, Ciarrocchi A, et al. Angiosarcoma and anaplastic carcinoma of the thyroid are two distinct entities: a morphologic, immunohistochemical, and genetic study. Mod Pathol. 2019; 32:787–98.
Article38. Colby T, Koss M, Travis W. Carcinoma of the lung; clinical, radiographic aspects, spread, staging, management, and prognosis. In: Colby T, Koss M, Travis W, eds. Tumors of the lower respiratory tract, 3rd series edition. Washington, DC: Armed Forces Institute of Pathology;1995. p. 107–34.39. Miettinen M, Franssila KO. Variable expression of keratins and nearly uniform lack of thyroid transcription factor 1 in thyroid anaplastic carcinoma. Hum Pathol. 2000; 31:1139–45.
Article40. Rossi G, Cavazza A, Sturm N, et al. Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: a clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol. 2003; 27:311–24.41. Moretti L, Medeiros LJ, Kunkalla K, Williams MD, Singh RR, Vega F. N-terminal PAX8 polyclonal antibody shows cross-reactivity with N-terminal region of PAX5 and is responsible for reports of PAX8 positivity in malignant lymphomas. Mod Pathol. 2012; 25:231–6.
Article42. Morgan EA, Pozdnyakova O, Nascimento AF, Hirsch MS. PAX8 and PAX5 are differentially expressed in B-cell and T-cell lymphomas. Histopathology. 2013; 62:406–13.
Article43. Lorenzo PI, Jimenez Moreno CM, Delgado I, et al. Immunohistochemical assessment of Pax8 expression during pancreatic islet development and in human neuroendocrine tumors. Histochem Cell Biol. 2011; 136:595–607.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Role of Claudin-1 Immunohistochemistry in Malignant Solid Tumors: A Meta-Analysis
- Meta-analysis
- Understanding the Meta-analysis; with an Example of the Meta: analysis Paper on the Therapeutic Effect of Variceal Bleeding
- Strengths and Limitations of Meta-Analysis
- Systematic review and meta-analysis of cancer risks in relation to environmental waste incinerator emissions: a meta-analysis of case-control and cohort studies