Int J Stem Cells.
2020 Jul;13(2):192-201. 10.15283/ijsc20044.
The Distinct Role of Tcfs and Lef1 in the Self-Renewal or Differentiation of Mouse Embryonic Stem Cells
- Affiliations
-
- 1Department of Life Science, University of Seoul, Seoul, Korea
- 2Asan Institute for Life Sciences, Seoul, Korea
- 3Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research at USC, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
- KMID: 2504332
- DOI: http://doi.org/10.15283/ijsc20044
Abstract
- Background and Objectives
Tcfs and Lef1 are DNA-binding transcriptional factors in the canonical Wnt signaling pathway. In the absence of β-catenin, Tcfs and Lef1 generally act as transcriptional repressors with co-repressor proteins such as Groucho, CtBP, and HIC-5. However, Tcfs and Lef1 turn into transcriptional activators during the interaction with β-catenin. Therefore, the activity of Tcfs and Lef1 is regulated by β-catenin. However, the intrinsic role of Tcfs and Lef1 has yet to be examined. The purpose of this study was to determine whether Tcfs and Lef1 play differential roles in the regulation of self-renewal and differentiation of mouse ES cells.
Methods and Results
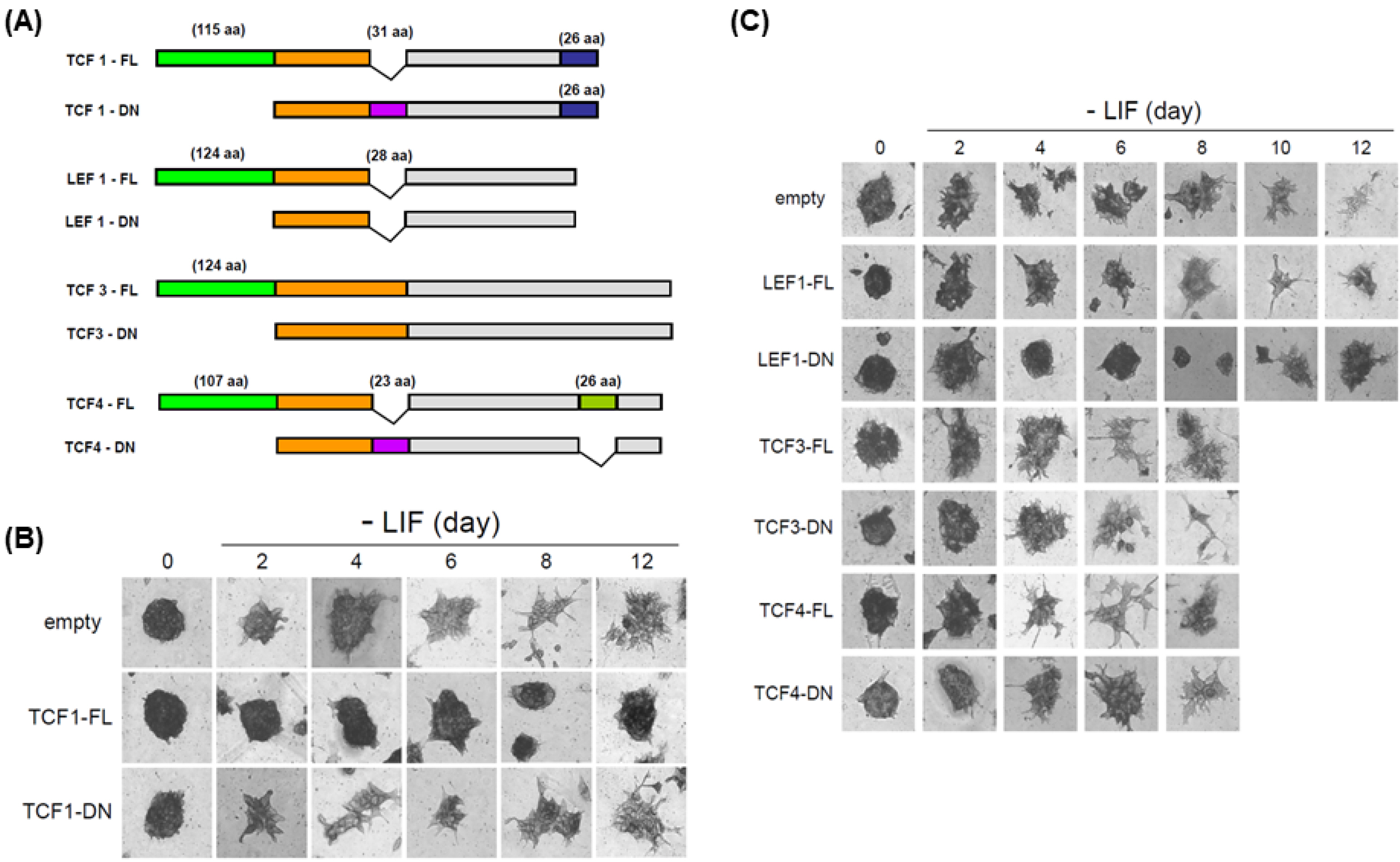
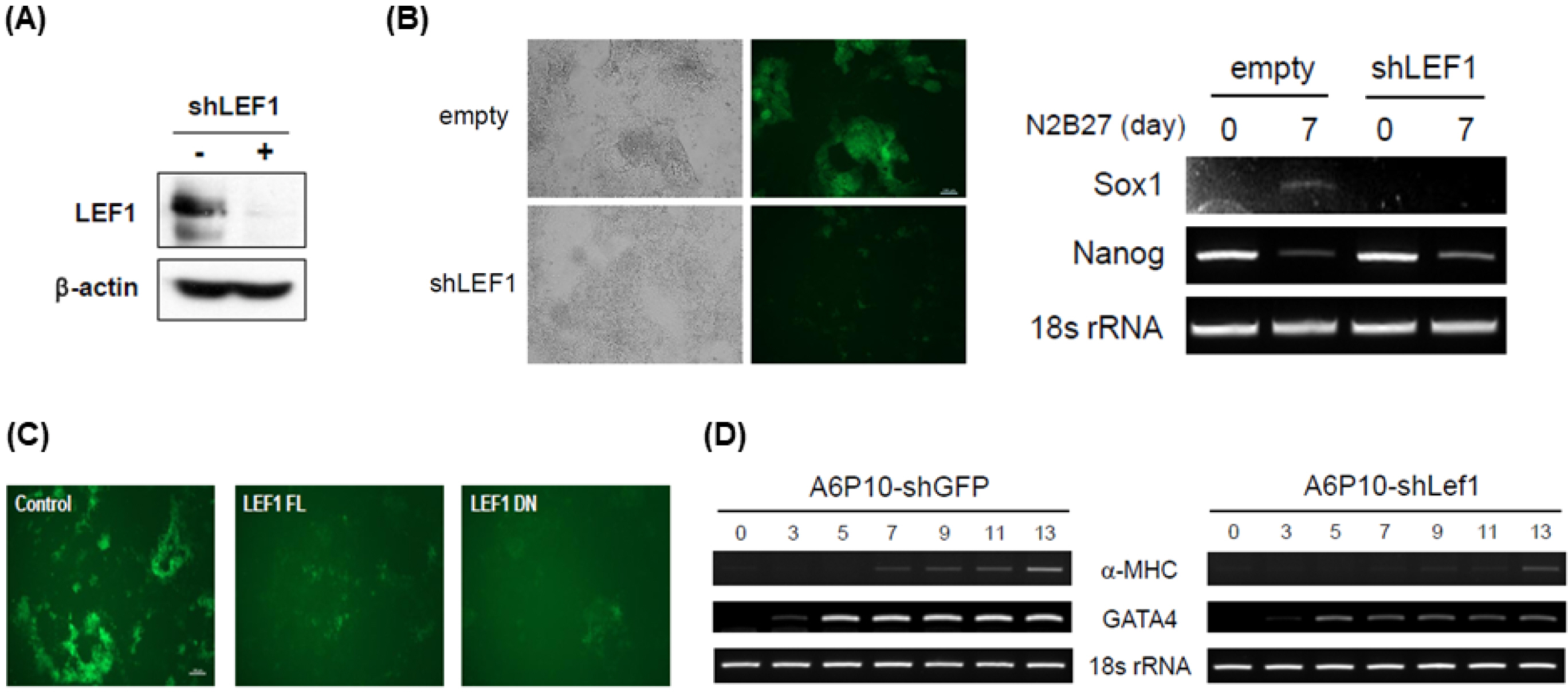
Interestingly, the expression of Tcfs and Lef1 was dynamically altered under various differentiation conditions, such as removal of LIF, EB formation and neuronal differentiation in N2B27 media, suggesting that the function of each Tcf and Lef1 may vary in ES cells. Ectopic expression of Tcf1 or the dominant negative form of Lef1 (Lef1-DN) contributes to ES cells to self-renew in the absence of leukemia inhibitory factor (LIF), whereas ectopic expression of Tcf3, Lef1 or Tcf1-DN did not support ES cells to self-renew. Ectopic expression of either Lef1 or Lef1-DN blocked neuronal differentiation, suggesting that the transient induction of Lef1 was necessary for the initiation and progress of differentiation. ChIP analysis shows that Tcf1 bound to Nanog promoter and ectopic expression of Tcf1 enhanced the transcription of Nanog.
Conclusions
The overall data suggest that Tcf1 plays a critical role in the maintenance of stemness whereas Lef1 is involved in the initiation of differentiation.
Keyword
Figure
Reference
-
References
1. Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. 2000; Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 18:399–404. DOI: 10.1038/74447. PMID: 10748519.
Article2. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. 1998; Embryonic stem cell lines derived from human blastocysts. Science. 282:1145–1147. DOI: 10.1126/science.282.5391.1145. PMID: 9804556.
Article3. Smith AG. 2001; Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 17:435–462. DOI: 10.1146/annurev.cellbio.17.1.435. PMID: 11687496.
Article4. Dahéron L, Opitz SL, Zaehres H, Lensch MW, Andrews PW, Itskovitz-Eldor J, Daley GQ. 2004; LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells. 22:770–778. DOI: 10.1634/stemcells.22-5-770. PMID: 15342941.
Article5. Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Schöler H, Smith A. 1998; Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 95:379–391. DOI: 10.1016/S0092-8674(00)81769-9.
Article6. Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. 2003; Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 17:126–140. DOI: 10.1101/gad.224503. PMID: 12514105. PMCID: PMC195970.
Article7. Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. 2003; Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 113:643–655. DOI: 10.1016/S0092-8674(03)00392-1. PMID: 12787505.
Article8. Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. 2003; The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 113:631–642. DOI: 10.1016/S0092-8674(03)00393-3. PMID: 12787504.
Article9. Niwa H, Miyazaki J, Smith AG. 2000; Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 24:372–376. DOI: 10.1038/74199. PMID: 10742100.
Article10. Reya T, Clevers H. 2005; Wnt signalling in stem cells and cancer. Nature. 434:843–850. DOI: 10.1038/nature03319. PMID: 15829953.
Article11. Merrill BJ. 2012; Wnt pathway regulation of embryonic stem cell self-renewal. Cold Spring Harb Perspect Biol. 4:a007971. DOI: 10.1101/cshperspect.a007971. PMID: 22952393. PMCID: PMC3428775.
Article12. Aubert J, Dunstan H, Chambers I, Smith A. 2002; Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat Biotechnol. 20:1240–1245. DOI: 10.1038/nbt763. PMID: 12447396.
Article13. Haegele L, Ingold B, Naumann H, Tabatabai G, Ledermann B, Brandner S. 2003; Wnt signalling inhibits neural differentiation of embryonic stem cells by controlling bone morphogenetic protein expression. Mol Cell Neurosci. 24:696–708. DOI: 10.1016/S1044-7431(03)00232-X. PMID: 14664819.
Article14. Kielman MF, Rindapää M, Gaspar C, van Poppel N, Breukel C, van Leeuwen S, Taketo MM, Roberts S, Smits R, Fodde R. 2002; Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. Nat Genet. 32:594–605. DOI: 10.1038/ng1045. PMID: 12426568.
Article15. Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. 2004; Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 10:55–63. DOI: 10.1038/nm979. PMID: 14702635.
Article16. Kirby LA, Schott JT, Noble BL, Mendez DC, Caseley PS, Peterson SC, Routledge TJ, Patel NV. 2012; Glycogen synthase kinase 3 (GSK3) inhibitor, SB-216763, promotes pluripotency in mouse embryonic stem cells. PLoS One. 7:e39329. DOI: 10.1371/journal.pone.0039329. PMID: 22745733. PMCID: PMC3383737.
Article17. Dravid G, Ye Z, Hammond H, Chen G, Pyle A, Donovan P, Yu X, Cheng L. 2005; Defining the role of Wnt/beta-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells. 23:1489–1501. DOI: 10.1634/stemcells.2005-0034. PMID: 16002782.
Article18. Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. 2007; Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci USA. 104:10894–10899. DOI: 10.1073/pnas.0704044104. PMID: 17576928. PMCID: PMC1904134.
Article19. Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. 2006; Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 133:3787–3796. DOI: 10.1242/dev.02551. PMID: 16943279.
Article20. Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. 2006; Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci USA. 103:19812–19817. DOI: 10.1073/pnas.0605768103. PMID: 17170140. PMCID: PMC1750922.
Article21. Otero JJ, Fu W, Kan L, Cuadra AE, Kessler JA. 2004; Beta-catenin signaling is required for neural differentiation of embryonic stem cells. Development. 131:3545–3557. DOI: 10.1242/dev.01218. PMID: 15262888.
Article22. Kim H, Kim S, Song Y, Kim W, Ying QL, Jho EH. 2015; Dual function of Wnt signaling during neuronal differentiation of mouse embryonic stem cells. Stem Cells Int. 2015:459301. DOI: 10.1155/2015/459301. PMID: 25945099. PMCID: PMC4402205.
Article23. Cadigan KM, Waterman ML. 2012; TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol. 4:a007906. DOI: 10.1101/cshperspect.a007906. PMID: 23024173. PMCID: PMC3536346.
Article24. Arce L, Yokoyama NN, Waterman ML. 2006; Diversity of LEF/TCF action in development and disease. Oncogene. 25:7492–7504. DOI: 10.1038/sj.onc.1210056. PMID: 17143293.
Article25. Hoppler S, Kavanagh CL. 2007; Wnt signalling: variety at the core. J Cell Sci. 120(Pt 3):385–393. DOI: 10.1242/jcs.03363. PMID: 17251379.
Article26. Hurlstone A, Clevers H. 2002; T-cell factors: turn-ons and turn-offs. EMBO J. 21:2303–2311. DOI: 10.1093/emboj/21.10.2303. PMID: 12006483. PMCID: PMC126013.27. Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. 2008; Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 22:746–755. DOI: 10.1101/gad.1642408. PMID: 18347094. PMCID: PMC2275428.
Article28. Pereira L, Yi F, Merrill BJ. 2006; Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell self-renewal. Mol Cell Biol. 26:7479–7491. DOI: 10.1128/MCB.00368-06. PMID: 16894029. PMCID: PMC1636872.
Article29. Tam WL, Lim CY, Han J, Zhang J, Ang YS, Ng HH, Yang H, Lim B. 2008; T-cell factor 3 regulates embryonic stem cell pluripotency and self-renewal by the transcriptional control of multiple lineage pathways. Stem Cells. 26:2019–2031. DOI: 10.1634/stemcells.2007-1115. PMID: 18467660. PMCID: PMC2692055.
Article30. Yi F, Pereira L, Merrill BJ. 2008; Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem Cells. 26:1951–1960. DOI: 10.1634/stemcells.2008-0229. PMID: 18483421. PMCID: PMC2743928.
Article31. Zhang Y, Zhu Z, Ding H, Wan S, Zhang X, Li Y, Ji J, Wang X, Zhang M, Ye SD. 2020; β-catenin stimulates Tcf7l1 degradation through recruitment of casein kinase 2 in mouse embryonic stem cells. Biochem Biophys Res Commun. 524:280–287. DOI: 10.1016/j.bbrc.2020.01.074. PMID: 31987502.
Article32. Ye S, Zhang T, Tong C, Zhou X, He K, Ban Q, Liu D, Ying QL. 2017; Depletion of Tcf3 and Lef1 maintains mouse embryonic stem cell self-renewal. Biol Open. 6:511–517. DOI: 10.1242/bio.022426. PMID: 28288968. PMCID: PMC5399551.
Article33. Ying QL, Stavridis M, Griffiths D, Li M, Smith A. 2003; Convertsion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 21:183–186. DOI: 10.1038/nbt780. PMID: 12524553.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pja2 Inhibits Wnt/β-catenin Signaling by Reducing the Level of TCF/LEF1
- HSP60 is required for stemness and proper differentiation of mouse embryonic stem cells
- Current Concepts of Stem Cell Therapy
- Cell Biological Characteristics of Adult Stem Cells
- Nuclear receptor regulation of stemness and stem cell differentiation