Korean J Transplant.
2020 Jun;34(2):100-108. 10.4285/kjt.2020.34.2.100.
Hepatocyte and mesenchymal stem cell co-transplantation in rats with acute liver failure
- Affiliations
-
- 1Department of Surgery, National Taiwan University Hospital, Taipei, Taiwan
- 2College of Medicine, National Taiwan University, Taipei, Taiwan
- 3Hepatitis Research Center, National Taiwan University Hospital, Taipei, Taiwan
- 4Department of Surgery, E-Da Hospital, I-Shou University, Kaohsiung, Taiwan
- KMID: 2503886
- DOI: http://doi.org/10.4285/kjt.2020.34.2.100
Abstract
- Background
Cell therapy is considered a potential alternative to liver transplantation in acute liver failure (ALF). We aimed to evaluate the add-on therapeutic benefit of hepatocyte and mesenchymal stem cell (MSC) cotransplantation over hepatocyte-only transplantations in a rat model of ALF.
Methods
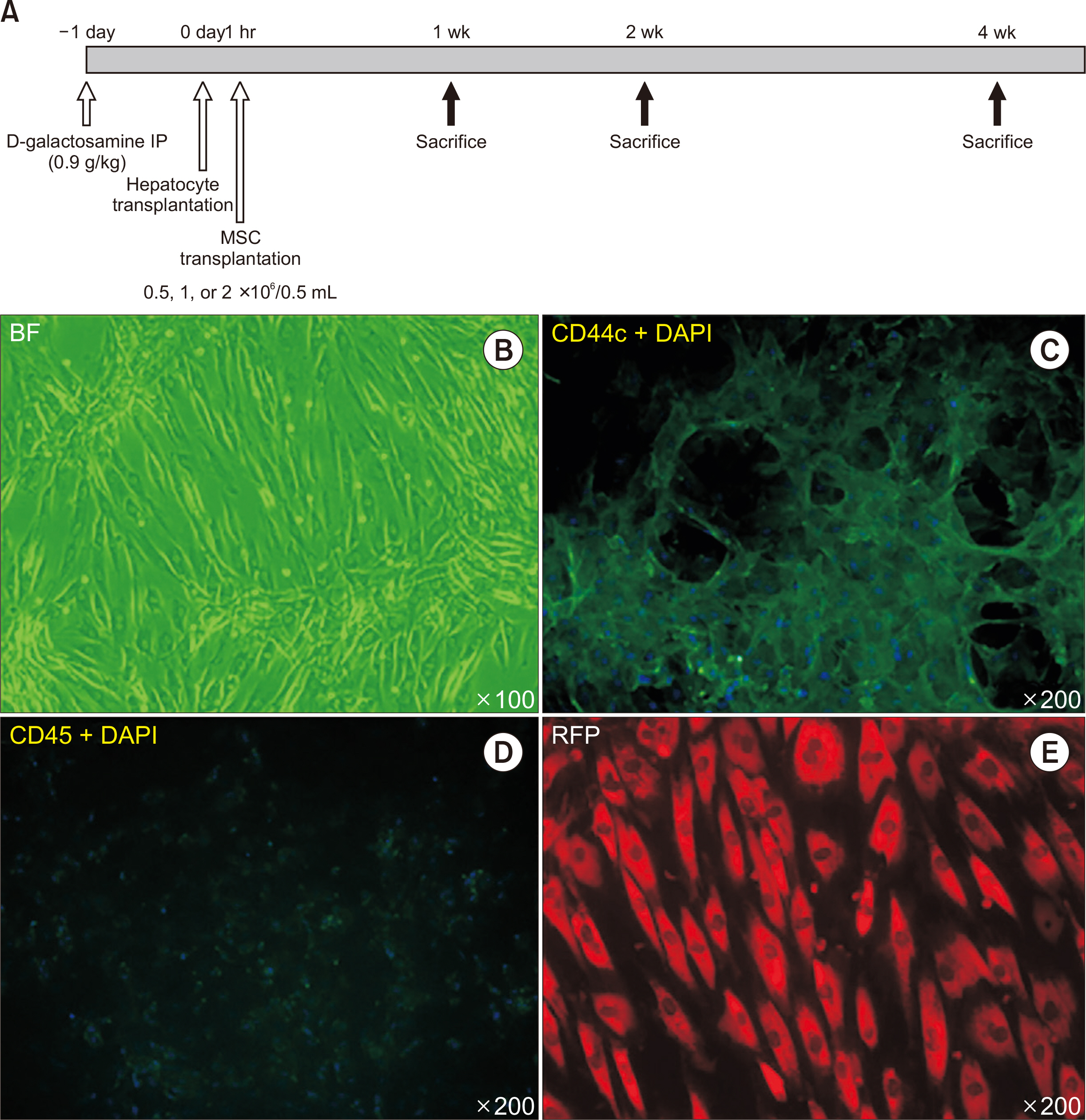
ALF was induced by D-galactosamine in Sprague-Dawley rats. Freshly isolated donor hepatocytes were derived from Tg (UBC-emGFP) rats and MSCs were collected from the bone marrow cells of DsRed rats. Donor hepatocytes (1×107/mL) were intraportally transplanted 24 hours after treatment with D-galactosamine over a 70-second interval, and donor MSCs (0.5, 1, or 2×106/0.5 mL) were intraportally transplanted 1 hour after the hepatocyte transplantation was complete. Animals were sacrificed after 7 and 14 days and subjected to donor cell identification, liver histology, serologic testing, and immunohistopathological examination.
Results
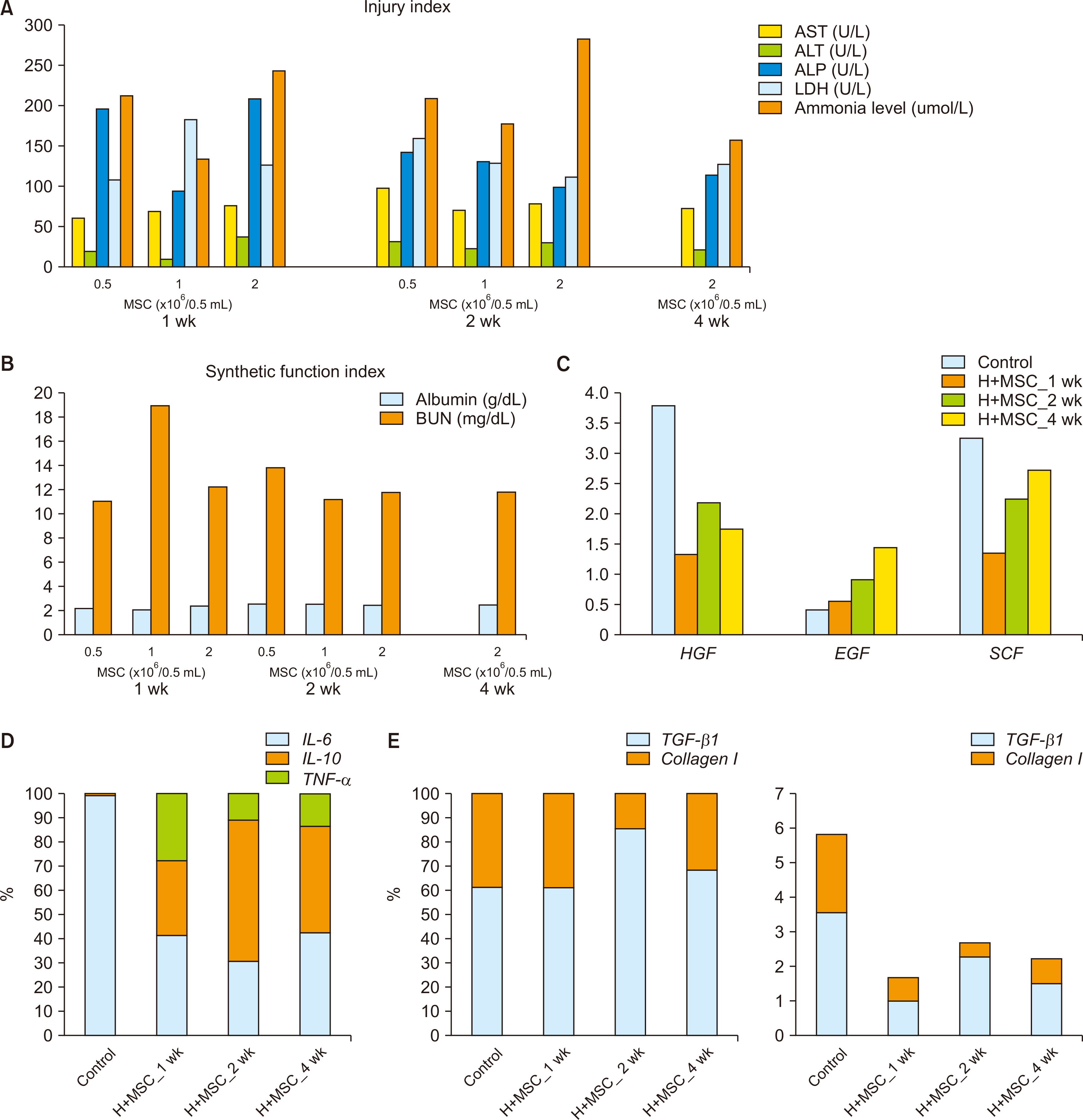
MSCs were observed in the periportal area, 1 and 2 weeks after transplantation. Transplanted hepatocytes did not actively proliferate when compared to hepatocyte-only transplantation. Morphologically, transplanted MSCs did not appear to differentiate into hepatocytes even 2 weeks after transplantation. Cotransplantation of MSCs was associated with lower macrophage infiltration, and reduced type I collagen, hepatocyte growth factor, tumor necrosis factor-α, and interleukin 10 expression, with similar gene expression profiles for epidermal growth factor and interleukin 6, when compared to hepatocyte-only transplantation.
Conclusions
Hepatocyte and MSC cotransplantation is feasible and safe in rat models of ALF. MSCs were found to survive the process and could be located within the periportal niches 2 weeks after treatment, without enhancing transplanted hepatocyte proliferation or differentiating into hepatocytes, while ameliorating the inflammatory response.
Figure
Reference
-
1. Iansante V, Mitry RR, Filippi C, Fitzpatrick E, Dhawan A. 2018; Human hepatocyte transplantation for liver disease: current status and future perspectives. Pediatr Res. 83:232–40. DOI: 10.1038/pr.2017.284. PMID: 29149103.
Article2. Soltys KA, Soto-Gutiérrez A, Nagaya M, Baskin KM, Deutsch M, Ito R, et al. 2010; Barriers to the successful treatment of liver disease by hepatocyte transplantation. J Hepatol. 53:769–74. DOI: 10.1016/j.jhep.2010.05.010. PMID: 20667616. PMCID: PMC2930077.
Article3. Donato MT, Lahoz A, Montero S, Bonora A, Pareja E, Mir J, et al. 2008; Functional assessment of the quality of human hepatocyte preparations for cell transplantation. Cell Transplant. 17:1211–9. DOI: 10.3727/096368908787236620. PMID: 19181215.
Article4. Strom SC, Chowdhury JR, Fox IJ. 1999; Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis. 19:39–48. DOI: 10.1055/s-2007-1007096. PMID: 10349682.
Article5. Ho CM, Chen YH, Chien CS, Ho YT, Ho SL, Hu RH, et al. 2015; Transplantation speed offers early hepatocyte engraftment in acute liver injured rats: a translational study with clinical implications. Liver Transpl. 21:652–61. DOI: 10.1002/lt.24106. PMID: 25821041.
Article6. Riordan SM, Williams R. 2000; Acute liver failure: targeted artificial and hepatocyte-based support of liver regeneration and reversal of multiorgan failure. J Hepatol. 32(1 Suppl):63–76. DOI: 10.1016/S0168-8278(00)80416-X. PMID: 10728795.
Article7. Zhang B, Inagaki M, Jiang B, Miyakoshi M, Arikura J, Ogawa K, et al. 2009; Effects of bone marrow and hepatocyte transplantation on liver injury. J Surg Res. 157:71–80. DOI: 10.1016/j.jss.2008.12.013. PMID: 19345373.
Article8. Gómez-Aristizábal A, Ng C, Ng J, Davies JE. 2012; Effects of two mesenchymal cell populations on hepatocytes and lymphocytes. Liver Transpl. 18:1384–94. DOI: 10.1002/lt.23500. PMID: 22753359.
Article9. van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, et al. 2008; Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 47:1634–43. DOI: 10.1002/hep.22236. PMID: 18395843.10. Balber AE. 2011; Concise review: aldehyde dehydrogenase bright stem and progenitor cell populations from normal tissues: characteristics, activities, and emerging uses in regenerative medicine. Stem Cells. 29:570–5. DOI: 10.1002/stem.613. PMID: 21308868.
Article11. Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, et al. 2007; Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 104:11002–7. DOI: 10.1073/pnas.0704421104. PMID: 17569781. PMCID: PMC1891813.
Article12. Hematti P. 2008; Role of mesenchymal stromal cells in solid organ transplantation. Transplant Rev (Orlando). 22:262–73. DOI: 10.1016/j.trre.2008.05.002. PMID: 18656340. PMCID: PMC2576746.
Article13. Gómez-Aristizábal A, Keating A, Davies JE. 2009; Mesenchymal stromal cells as supportive cells for hepatocytes. Mol Ther. 17:1504–8. DOI: 10.1038/mt.2009.158. PMID: 19584815. PMCID: PMC2835270.
Article14. Yagi H, Parekkadan B, Suganuma K, Soto-Gutierrez A, Tompkins RG, Tilles AW, et al. 2009; Long-term superior performance of a stem cell/hepatocyte device for the treatment of acute liver failure. Tissue Eng Part A. 15:3377–88. DOI: 10.1089/ten.tea.2008.0681. PMID: 19397469. PMCID: PMC2792056.
Article15. Meier RP, Müller YD, Morel P, Gonelle-Gispert C, Bühler LH. 2013; Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence? Stem Cell Res. 11:1348–64. DOI: 10.1016/j.scr.2013.08.011. PMID: 24090934.
Article16. Liu WH, Song FQ, Ren LN, Guo WQ, Wang T, Feng YX, et al. 2015; The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases. J Cell Mol Med. 19:511–20. DOI: 10.1111/jcmm.12482. PMID: 25534251. PMCID: PMC4369809.
Article17. Kanazawa H, Fujimoto Y, Teratani T, Iwasaki J, Kasahara N, Negishi K, et al. 2011; Bone marrow-derived mesenchymal stem cells ameliorate hepatic ischemia reperfusion injury in a rat model. PLoS One. 6:e19195. DOI: 10.1371/journal.pone.0019195. PMID: 21559442. PMCID: PMC3084802.
Article18. Li J, Zhang L, Xin J, Jiang L, Li J, Zhang T, et al. 2012; Immediate intraportal transplantation of human bone marrow mesenchymal stem cells prevents death from fulminant hepatic failure in pigs. Hepatology. 56:1044–52. DOI: 10.1002/hep.25722. PMID: 22422600.
Article19. Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, et al. 2012; Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 307:1169–77. DOI: 10.1001/jama.2012.316. PMID: 22436957.20. Wan CD, Cheng R, Wang HB, Liu T. 2008; Immunomodulatory effects of mesenchymal stem cells derived from adipose tissues in a rat orthotopic liver transplantation model. Hepatobiliary Pancreat Dis Int. 7:29–33.21. Baertschiger RM, Serre-Beinier V, Morel P, Bosco D, Peyrou M, Clément S, et al. 2009; Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One. 4:e6657. DOI: 10.1371/journal.pone.0006657. PMID: 19684854. PMCID: PMC2722022.
Article22. Yu CH, Chen HL, Chen WT, Ni YH, Lin YL, Chang MH. 2004; Portal hemodynamic changes after hepatocyte transplantation in acute hepatic failure. J Biomed Sci. 11:756–63. DOI: 10.1007/BF02254360. PMID: 15591772.
Article23. Rajvanshi P, Kerr A, Bhargava KK, Burk RD, Gupta S. 1996; Studies of liver repopulation using the dipeptidyl peptidase IV-deficient rat and other rodent recipients: cell size and structure relationships regulate capacity for increased transplanted hepatocyte mass in the liver lobule. Hepatology. 23:482–96. DOI: 10.1002/hep.510230313. PMID: 8617428.
Article24. Soleimani M, Nadri S. 2009; A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 4:102–6. DOI: 10.1038/nprot.2008.221. PMID: 19131962.
Article25. Yu CH, Chen HL, Chen YH, Chang MF, Chien CS, Chang MH. 2009; Impaired hepatocyte regeneration in acute severe hepatic injury enhances effective repopulation by transplanted hepatocytes. Cell Transplant. 18:1081–92. DOI: 10.3727/096368909X12483162196647. PMID: 19650970.
Article26. Schäfer R, Spohn G, Baer PC. 2016; Mesenchymal stem/stromal cells in regenerative medicine: can preconditioning strategies improve therapeutic efficacy? Transfus Med Hemother. 43:256–67. DOI: 10.1159/000447458. PMID: 27721701. PMCID: PMC5040900.
Article27. Wang J, Ren H, Yuan X, Ma H, Shi X, Ding Y. 2018; Interleukin-10 secreted by mesenchymal stem cells attenuates acute liver failure through inhibiting pyroptosis. Hepatol Res. 48:E194–202. DOI: 10.1111/hepr.12969. PMID: 28833919.
Article28. Lee SC, Kim KH, Kim OH, Lee SK, Hong HE, Won SS, et al. 2017; Determination of optimized oxygen partial pressure to maximize the liver regenerative potential of the secretome obtained from adipose-derived stem cells. Stem Cell Res Ther. 8:181. DOI: 10.1186/s13287-017-0635-x. PMID: 28774345. PMCID: PMC5543744.
Article29. Possamai LA, Thursz MR, Wendon JA, Antoniades CG. 2014; Modulation of monocyte/macrophage function: a therapeutic strategy in the treatment of acute liver failure. J Hepatol. 61:439–45. DOI: 10.1016/j.jhep.2014.03.031. PMID: 24703954.
Article30. Galipeau J, Sensébé L. 2018; Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 22:824–33. DOI: 10.1016/j.stem.2018.05.004. PMID: 29859173. PMCID: PMC6434696.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mesenchymal Stem Cell Therapy in Acute Liver Failure
- Hepatic Stem Cell Transplantation in Liver Failure
- Liver Stem Cells
- Advanced Research on Stem Cell Therapy for Hepatic Diseases: Potential Implications of a Placenta-derived Mesenchymal Stem Cell-based Strategy
- Clinical application of stem cells in liver diseases