Korean J Transplant.
2020 Jun;34(2):84-91. 10.4285/kjt.2020.34.2.84.
Long-term compensation of renal function after donor nephrectomy
- Affiliations
-
- 1Department of Medicine, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Surgery, Yonsei University College of Medicine, Seoul, Korea
- 3The Research Institute for Transplantation, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2503884
- DOI: http://doi.org/10.4285/kjt.2020.34.2.84
Abstract
- Background
Living donors are the major source of kidneys in countries with a shortage of deceased donors. Kidney donation after careful donor selection is generally accepted as a safe procedure, but the physiologic consequences after donor nephrectomy are not fully verified. In this study we retrospectively reviewed the renal function of the residual kidney in living donors.
Methods
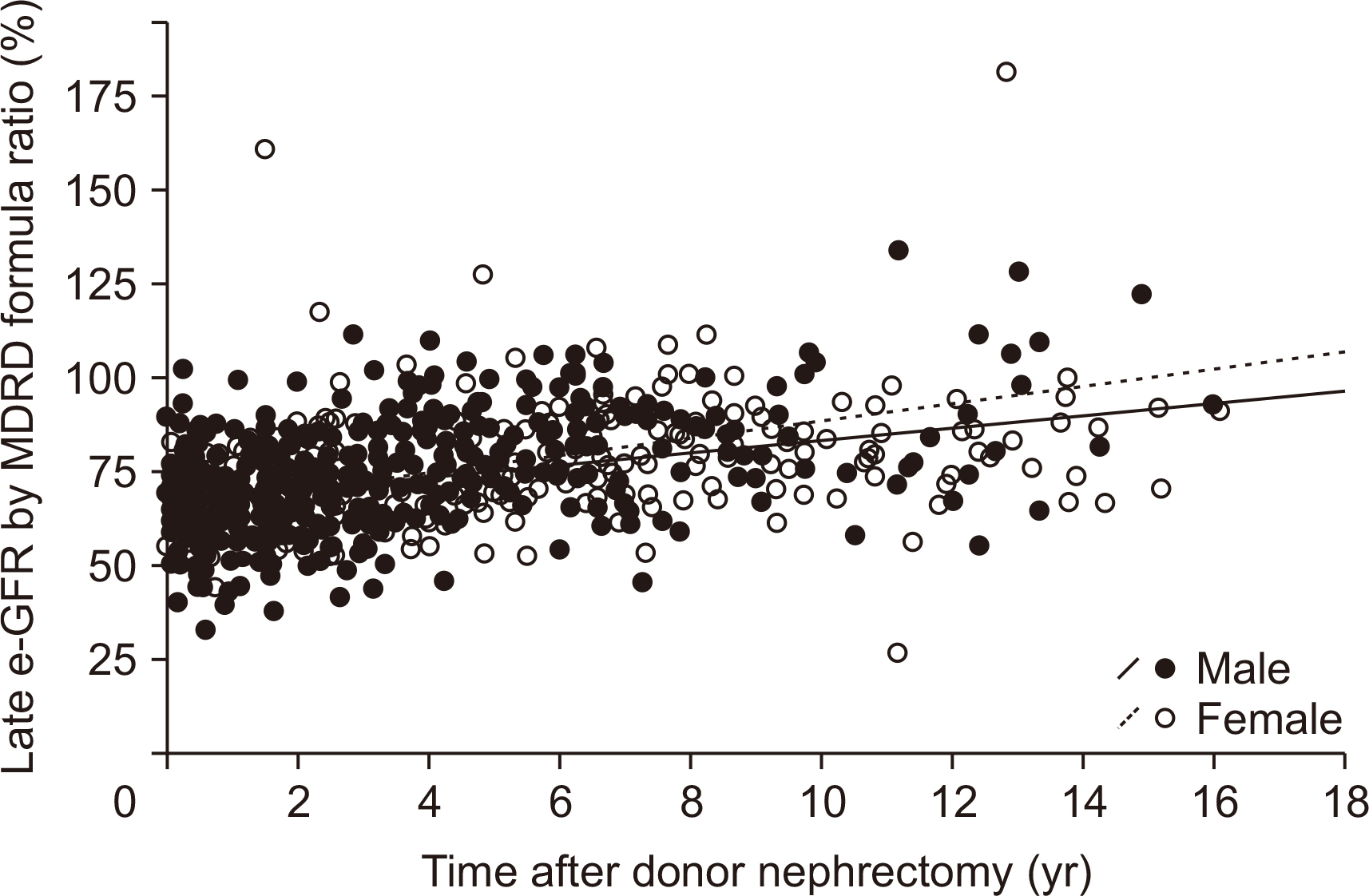
Post-nephrectomy laboratory data of 1,175 living donors (60.7%) from 1,933 living donors who received uninephrectomy from January 1999 to December 2017 at Yonsei University, Severance Hospital, Korea were retrospectively collected. Post-nephrectomy renal function was monitored by the relative ratio of estimated glomerular filtration rate (e-GFR; pre-nephrectomy e-GFR ratio vs. post-nephrectomy e-GFR) that was calculated by the Modification of Diet in Renal Disease formula.
Results
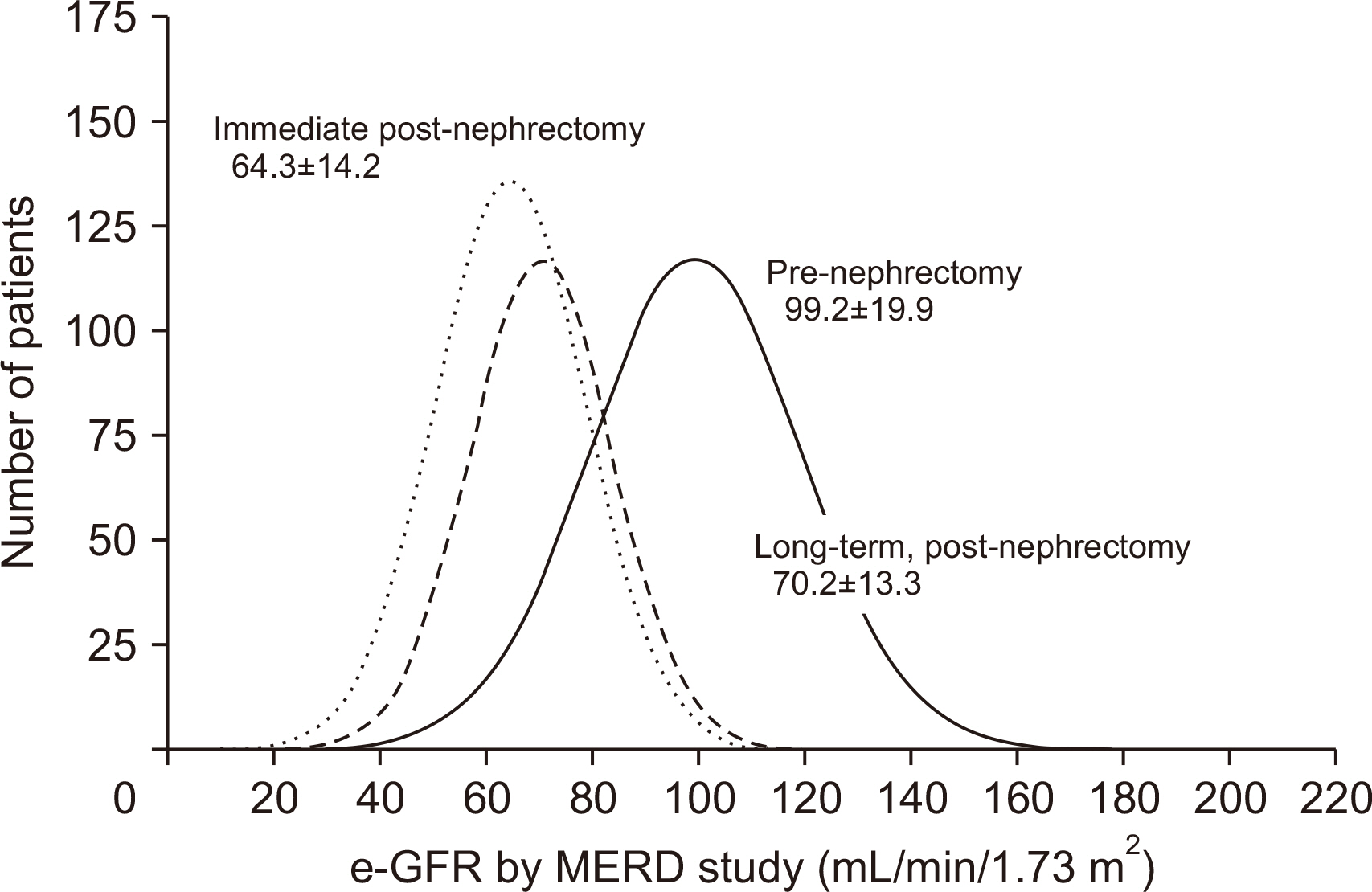
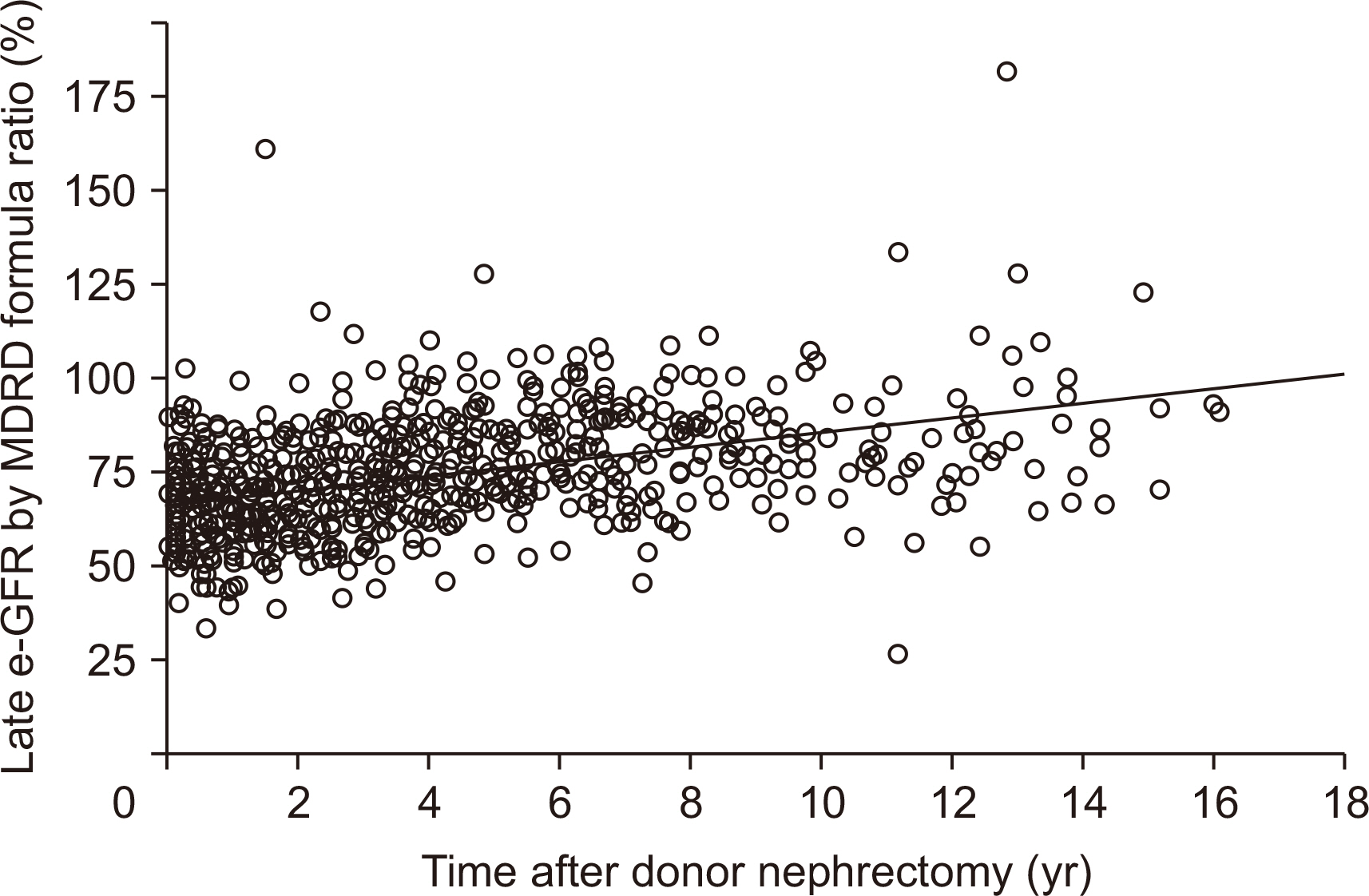
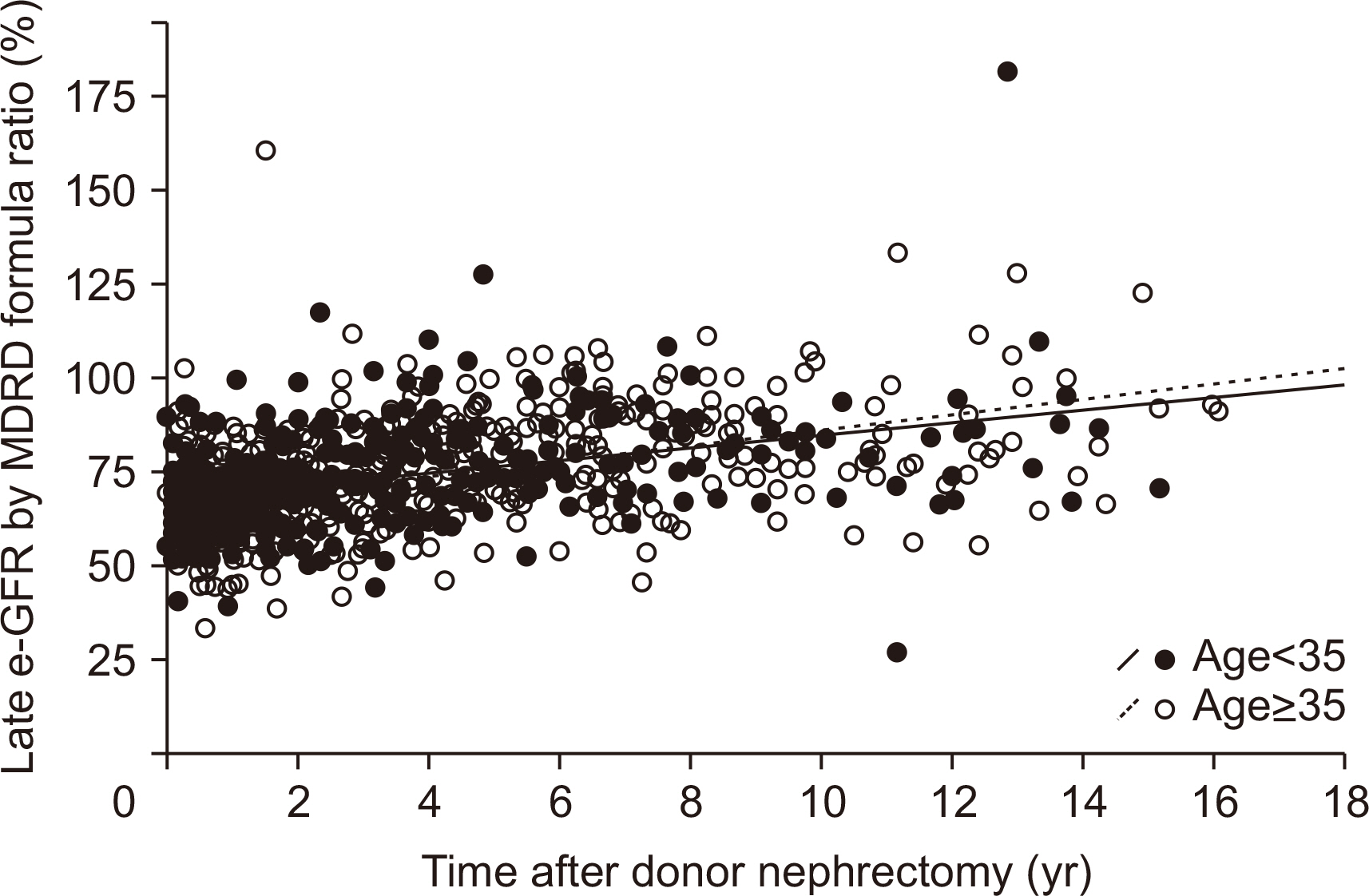
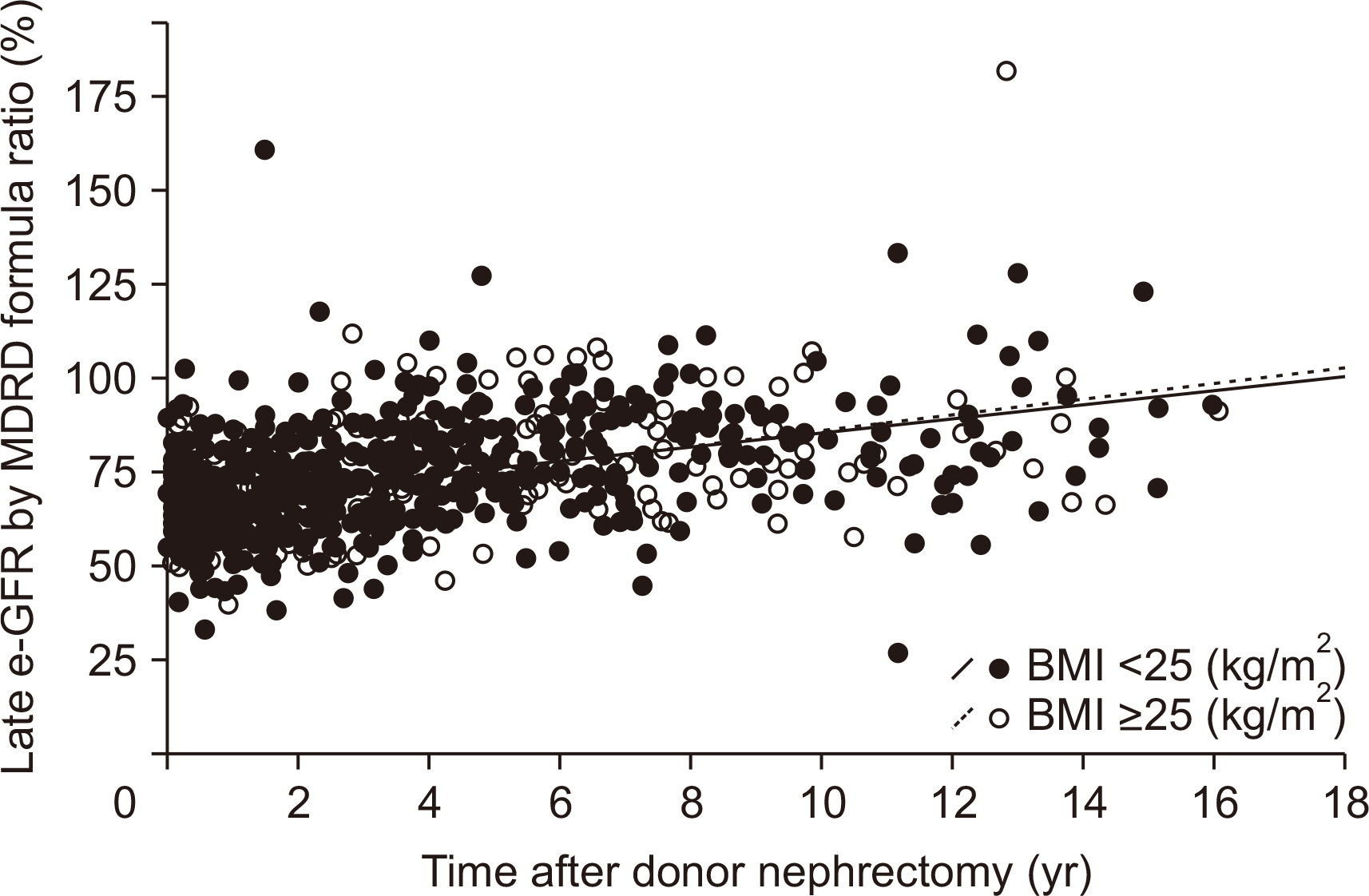
During 36.3±37.6 months of mean follow-up, two cases (0.17%, 2/1,175) of renal failure developed. The mean e-GFR decreased to 64.3±14.2 mL/min/1.73 m2 immediately after nephrectomy from 99.2±19.9 mL/min/1.73 m2 of the pre-nephrectomy e-GFR. Early decrement of e-GFR was prominent in male and obese donors (body mass index >25 kg/m2, P<0.05). The e-GFR ratio increased according to post-nephrectomy duration, and the mean increment degree of e-GFR ratio after nephrectomy calculated by linear regression analysis was 1.94% per year. Unlike the early decrement of e-GFR ratio after nephrectomy, donor factors such as degree of obesity and donor sex did not affect the late increment of e-GFR ratio after nephrectomy (P>0.05).
Conclusions
Our data showed that long-term compensation of the renal function after nephrectomy occurs independently of preoperative donor characteristics.
Figure
Reference
-
1. Korean Network for Organ Sharing (KONOS). 2020. 2017 Annual data report [Internet]. KONOS;Seoul: Available from: http://www.konos.go.kr. cited 2020 May 27.2. United States Renal Data System. 2017. USRDS 2017 annual data report [Internet]. Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases;Bethesda (MD): Available from https://www.usrds.org/2017/view/. cited 2020 May 27.3. Gaston RS, Kumar V, Matas AJ. 2015; Reassessing medical risk in living kidney donors. J Am Soc Nephrol. 26:1017–9. DOI: 10.1681/ASN.2014030227. PMID: 25255922. PMCID: PMC4413760.
Article4. Edgren J, Laasonen L, Kock B, Brotherus JW, Pasternack A, Kuhlbäck B. 1976; Kidney function and compensatory growth of the kidney in living kidney donors. Scand J Urol Nephrol. 10:134–6. DOI: 10.3109/00365597609179673. PMID: 948722.
Article5. Orecklin JR, Craven JD, Lecky JW. 1973; Compensatory renal hypertrophy: a morphologic study in transplant donors. J Urol. 109:952–4. DOI: 10.1016/S0022-5347(17)60591-3. PMID: 4575812.
Article6. Takagi T, Mir MC, Sharma N, Remer EM, Li J, Demirjian S, et al. 2014; Compensatory hypertrophy after partial and radical nephrectomy in adults. J Urol. 192:1612–8. DOI: 10.1016/j.juro.2014.06.018. PMID: 24931802.
Article7. Saxena AB, Myers BD, Derby G, Blouch KL, Yan J, Ho B, et al. 2006; Adaptive hyperfiltration in the aging kidney after contralateral nephrectomy. Am J Physiol Renal Physiol. 291:F629–34. DOI: 10.1152/ajprenal.00329.2005. PMID: 16525160.
Article8. Srivastava T, Hariharan S, Alon US, McCarthy ET, Sharma R, El-Meanawy A, et al. 2018; Hyperfiltration-mediated injury in the remaining kidney of a transplant donor. Transplantation. 102:1624–35. DOI: 10.1097/TP.0000000000002304. PMID: 29847501. PMCID: PMC6153061.
Article9. Praga M. 2005; Synergy of low nephron number and obesity: a new focus on hyperfiltration nephropathy. Nephrol Dial Transplant. 20:2594–7. DOI: 10.1093/ndt/gfi201. PMID: 16223782.
Article10. Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. 2001; Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. J Am Soc Nephrol. 12:1315–25. PMID: 11373357.
Article11. Mjøen G, Hallan S, Hartmann A, Foss A, Midtvedt K, Øyen O, et al. 2014; Long-term risks for kidney donors. Kidney Int. 86:162–7. DOI: 10.1038/ki.2013.460. PMID: 24284516.
Article12. González E, Gutiérrez E, Morales E, Hernández E, Andres A, Bello I, et al. 2005; Factors influencing the progression of renal damage in patients with unilateral renal agenesis and remnant kidney. Kidney Int. 68:263–70. DOI: 10.1111/j.1523-1755.2005.00401.x. PMID: 15954916.13. Locke JE, Reed RD, Massie A, MacLennan PA, Sawinski D, Kumar V, et al. 2017; Obesity increases the risk of end-stage renal disease among living kidney donors. Kidney Int. 91:699–703. DOI: 10.1016/j.kint.2016.10.014. PMID: 28041626. PMCID: PMC5313335.
Article14. Ito K, Nakashima J, Hanawa Y, Oya M, Ohigashi T, Marumo K, et al. 2004; The prediction of renal function 6 years after unilateral nephrectomy using preoperative risk factors. J Urol. 171:120–5. DOI: 10.1097/01.ju.0000100981.11470.2f. PMID: 14665858.
Article15. Esposito C, Plati A, Mazzullo T, Fasoli G, De Mauri A, Grosjean F, et al. 2007; Renal function and functional reserve in healthy elderly individuals. J Nephrol. 20:617–25. PMID: 17918149.16. Rook M, Bosma RJ, van Son WJ, Hofker HS, van der Heide JJ, ter Wee PM, et al. 2008; Nephrectomy elicits impact of age and BMI on renal hemodynamics: lower postdonation reserve capacity in older or overweight kidney donors. Am J Transplant. 8:2077–85. DOI: 10.1111/j.1600-6143.2008.02355.x. PMID: 18727700.
Article17. Fehrman-Ekholm I, Dunér F, Brink B, Tydén G, Elinder CG. 2001; No evidence of accelerated loss of kidney function in living kidney donors: results from a cross-sectional follow-up. Transplantation. 72:444–9. DOI: 10.1097/00007890-200108150-00015. PMID: 11502974.18. Lam NN, Lentine KL, Levey AS, Kasiske BL, Garg AX. 2015; Long-term medical risks to the living kidney donor. Nat Rev Nephrol. 11:411–9. DOI: 10.1038/nrneph.2015.58. PMID: 25941060.
Article19. O'Keeffe LM, Ramond A, Oliver-Williams C, Willeit P, Paige E, Trotter P, et al. 2018; Mid- and long-term health risks in living kidney donors: a systematic review and meta-analysis. Ann Intern Med. 168:276–84. DOI: 10.7326/M17-1235. PMID: 29379948.20. Kasiske BL, Ma JZ, Louis TA, Swan SK. 1995; Long-term effects of reduced renal mass in humans. Kidney Int. 48:814–9. DOI: 10.1038/ki.1995.355. PMID: 7474669.
Article21. Kasiske BL, Anderson-Haag T, Israni AK, Kalil RS, Kimmel PL, Kraus ES, et al. 2015; A prospective controlled study of living kidney donors: three-year follow-up. Am J Kidney Dis. 66:114–24. DOI: 10.1053/j.ajkd.2015.01.019. PMID: 25795073. PMCID: PMC4485526.
Article22. Seo MH, Lee WY, Kim SS, Kang JH, Kang JH, Kim KK, et al. 2019; 2018 Korean Society for the Study of Obesity guideline for the management of obesity in Korea. J Obes Metab Syndr. 28:40–5. DOI: 10.7570/jomes.2019.28.1.40. PMID: 31089578. PMCID: PMC6484940.
Article23. Pottel H, Delanaye P, Weekers L, Selistre L, Goffin K, Gheysens O, et al. 2017; Age-dependent reference intervals for estimated and measured glomerular filtration rate. Clin Kidney J. 10:545–51. DOI: 10.1093/ckj/sfx026. PMID: 28852494. PMCID: PMC5570001.
Article24. Weitz J, Koch M, Mehrabi A, Schemmer P, Zeier M, Beimler J, et al. 2006; Living-donor kidney transplantation: risks of the donor: benefits of the recipient. Clin Transplant. 20(Suppl 17):13–6. DOI: 10.1111/j.1399-0012.2006.00595.x. PMID: 17100696.25. Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, et al. 2009; Long-term consequences of kidney donation. N Engl J Med. 360:459–69. DOI: 10.1056/NEJMoa0804883. PMID: 19179315. PMCID: PMC3559132.
Article26. Rizvi SA, Naqvi SA, Jawad F, Ahmed E, Asghar A, Zafar MN, et al. 2005; Living kidney donor follow-up in a dedicated clinic. Transplantation. 79:1247–51. DOI: 10.1097/01.TP.0000161666.05236.97. PMID: 15880079.
Article27. Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL, et al. 2014; Risk of end-stage renal disease following live kidney donation. JAMA. 311:579–86. DOI: 10.1001/jama.2013.285141. PMID: 24519297. PMCID: PMC4411956.
Article28. Hartmann A, Fauchald P, Westlie L, Brekke IB, Holdaas H. 2003; The risk of living kidney donation. Nephrol Dial Transplant. 18:871–3. DOI: 10.1093/ndt/gfg069. PMID: 12686656.
Article29. ter Wee PM, Tegzess AM, Donker AJ. 1994; Pair-tested renal reserve filtration capacity in kidney recipients and their donors. J Am Soc Nephrol. 4:1798–808. PMID: 8068878.
Article30. Rook M, Hofker HS, van Son WJ, Homan van der Heide JJ, Ploeg RJ, Navis GJ. 2006; Predictive capacity of pre-donation GFR and renal reserve capacity for donor renal function after living kidney donation. Am J Transplant. 6:1653–9. DOI: 10.1111/j.1600-6143.2006.01359.x. PMID: 16827867.
Article31. Goldfarb DA, Matin SF, Braun WE, Schreiber MJ, Mastroianni B, Papajcik D, et al. 2001; Renal outcome 25 years after donor nephrectomy. J Urol. 166:2043–7. DOI: 10.1016/S0022-5347(05)65502-4. PMID: 11696703.
Article32. Gossmann J, Wilhelm A, Kachel HG, Jordan J, Sann U, Geiger H, et al. 2005; Long-term consequences of live kidney donation follow-up in 93% of living kidney donors in a single transplant center. Am J Transplant. 5:2417–24. DOI: 10.1111/j.1600-6143.2005.01037.x. PMID: 16162190.
Article33. Garg AX, Muirhead N, Knoll G, Yang RC, Prasad GV, Thiessen-Philbrook H, et al. 2006; Proteinuria and reduced kidney function in living kidney donors: a systematic review, meta-analysis, and meta-regression. Kidney Int. 70:1801–10. DOI: 10.1038/sj.ki.5001819. PMID: 17003822.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term Change of Renal Function after Donor Nephrectomy for Kidney Transplantation
- Changes of renal function in the remaining kidney after donor nephrectomy
- Short-term Follow-up of Renal Function after Donor Nephrectomy
- Living Donor Nephrectomy: Surgical Selection Criteria of Laterality
- Living Donor Nephrectomy