Ann Pediatr Endocrinol Metab.
2020 Jun;25(2):104-111. 10.6065/apem.1938156.078.
Serum uric acid in Korean children and adolescents: reference percentiles and association with metabolic syndrome
- Affiliations
-
- 1Depar tment of Pediatrics, Korea Cancer Center Hospital, Seoul, Korea
- KMID: 2503361
- DOI: http://doi.org/10.6065/apem.1938156.078
Abstract
- Purpose
To establish age/sex-specific reference intervals for serum uric acid and to examine the associations between serum uric acid level and metabolic syndrome (MetS) and its components in Korean children and adolescents.
Methods
We analyzed data for 1,349 subjects aged 10 to 19 years from the Korea National Health and Nutrition Examination Survey 2016–2017.
Results
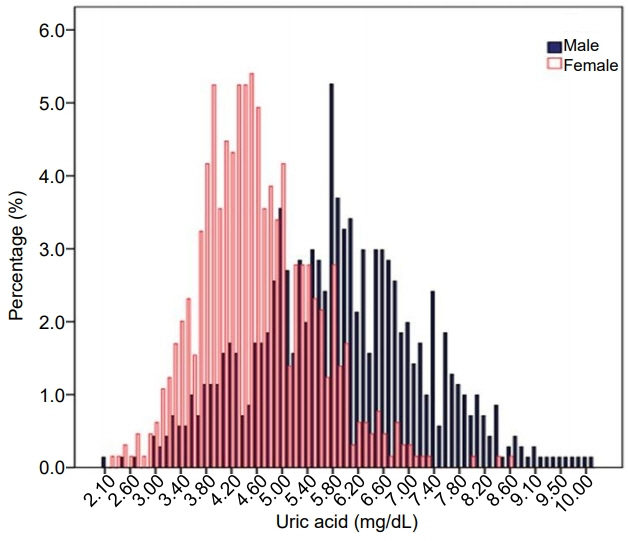
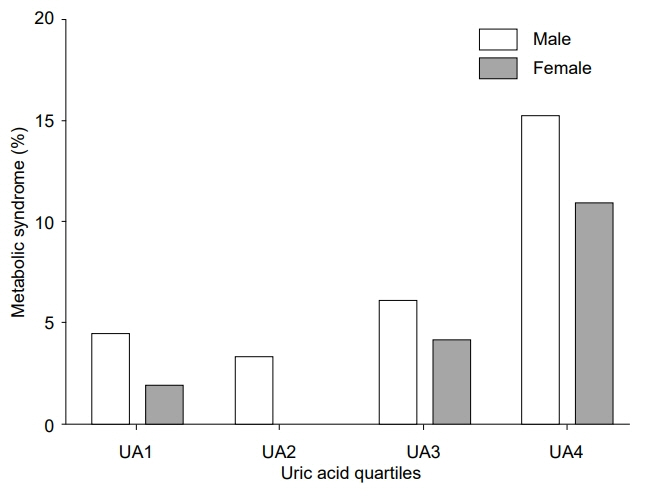
The mean uric acid levels were 5.9±1.3 mg/dL (interquartile range, 5.0–6.8 mg/dL) in males and 4.6±0.9 mg/dL (interquartile range, 3.9–5.2 mg/dL) in females. The mean uric acid level increased significantly from 10–13 years of age in males, but not in females. The overall prevalence of MetS was 5.9% (7.3% in males and 4.3% in females; P=0.022). The prevalences of MetS in the lowest, second, third, and highest quartiles of uric acid level were 4.4%, 3.3%, 6.1%, and 15.2%, respectively, in males (P for trend <0.001) and 1.9%, 0.0%, 4.1%, and 10.9%, respectively, in females (P for trend <0.001). Compared with the lowest quartile of uric acid level, the odds ratio (with 95% confidence interval) for MetS in the highest quartile was 2.897 (1.140–7.361) in males and 5.173 (1.459–18.342) in females. Subjects in the highest quartile exhibited increased risk for abdominal obesity and low high-density lipoprotein cholesterol in both sexes.
Conclusion
Serum uric acid level is positively associated with MetS and its components abdominal obesity and low high-density lipoprotein cholesterol.
Keyword
Figure
Cited by 2 articles
-
Metabolic Syndrome in Children and Adolescents
Yoojin Lindsey Chung, Young-Jun Rhie
Ewha Med J. 2022;45(4):e13. doi: 10.12771/emj.2022.e13.Association between serum uric acid and kidney disease with pediatric focus
Myung Hyun Cho
Child Kidney Dis. 2024;28(3):106-111. doi: 10.3339/ckd.24.016.
Reference
-
References
1. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005; 365:1415–28.
Article2. Duncan GE, Li SM, Zhou XH. Prevalence and trends of a metabolic syndrome phenotype among u.s. Adolescents, 1999-2000. Diabetes Care. 2004; 27:2438–43.
Article3. Kim S, So WY. Prevalence of Metabolic Syndrome among Korean Adolescents According to the National Cholesterol Education Program, Adult Treatment Panel III and International Diabetes Federation. Nutrients. 2016; 8:588.
Article4. Choi KM, Kim SM, Kim YE, Choi DS, Baik SH, Lee J, et al. Prevalence and cardiovascular disease risk of the metabolic syndrome using National Cholesterol Education Program and International Diabetes Federation definitions in the Korean population. Metabolism. 2007; 56:552–8.
Article5. Dunstan DW, Zimmet PZ, Welborn TA, De Courten MP, Cameron AJ, Sicree RA, et al. The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care. 2002; 25:829–34.6. Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008; 152:201–6.
Article7. Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001; 24:683–9.
Article8. Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis. 2004; 173:309–14.
Article9. Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016; 213:8–14.
Article10. de Oliveira EP, Burini RC. High plasma uric acid concentration: causes and consequences. Diabetol Metab Syndr. 2012; 4:12.
Article11. Borghi C, Verardi FM, Pareo I, Bentivenga C, Cicero AF. Hyperuricemia and cardiovascular disease risk. Expert Rev Cardiovasc Ther. 2014; 12:1219–25.
Article12. Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007; 115:2526–32.
Article13. Sun HL, Pei D, Lue KH, Chen YL. Uric acid levels can predict metabolic syndrome and hypertension in adolescents: a 10-year longitudinal study. PLoS One. 2015; 10:e0143786.
Article14. Bhole V, Choi JW, Kim SW, de Vera M, Choi H. Serum uric acid levels and the risk of type 2 diabetes: a prospective study. Am J Med. 2010; 123:957–61.
Article15. Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea National Health and Nutrition Examination Sur vey (KNHANES). Int J Epidemiol. 2014; 43:69–77.
Article16. Yi KH, Hwang JS, Kim EY, Lee SH, Kim DH, Lim JS. Prevalence of insulin resistance and cardiometabolic risk in Korean children and adolescents: a population-based study. Diabetes Res Clin Pract. 2014; 103:106–13.
Article17. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499–502.
Article18. Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med. 2003; 157:821–7.
Article19. Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, et al. 2007 Korean National Growth Charts: review of developmental process and an outlook. Korean J Pediatr. 2008; 51:1–25.
Article20. Shatat IF, Abdallah RT, Sas DJ, Hailpern SM. Serum uric acid in U.S. adolescents: distribution and relationship to demographic characteristics and cardiovascular risk factors. Pediatr Res. 2012; 72:95–100.
Article21. Wang ZN, Li P, Jiang RH, Li L, Li X, Li L, et al. The association between serum uric acid and metabolic syndrome among adolescents in northeast China. Int J Clin Exp Med. 2015; 8:21122–9.22. Southcott EK, Kerrigan JL, Potter JM, Telford RD, Waring P, Reynolds GJ, et al. Establishment of pediatric reference intervals on a large cohort of healthy children. Clin Chim Acta. 2010; 411:1421–7.
Article23. Cho SM, Lee SG, Kim HS, Kim JH. Establishing pediatric reference intervals for 13 biochemical analytes derived from normal subjects in a pediatric endocrinology clinic in Korea. Clin Biochem. 2014; 47:268–71.
Article24. Lee MS, Wahlqvist ML, Yu HL, Pan WH. Hyperuricemia and metabolic syndrome in Taiwanese children. Asia Pac J Clin Nutr. 2007; 16 Suppl 2:594–600.25. Choi H, Kim HC, Song BM, Park JH, Lee JM, Yoon DL, et al. Serum uric acid concentration and metabolic syndrome among elderly Koreans: The Korean Urban Rural Elderly (KURE) study. Arch Gerontol Geriatr. 2016; 64:51–8.
Article26. Wang JY, Chen YL, Hsu CH, Tang SH, Wu CZ, Pei D. Predictive value of serum uric acid levels for the diagnosis of metabolic syndrome in adolescents. J Pediatr. 2012; 161:753–6. e2.
Article27. Ferrannini E, Mari A. Beta cell function and its relation to insulin action in humans: a critical appraisal. Diabetologia. 2004; 47:943–56.
Article28. Ighbariya A, Weiss R. Insulin resistance, prediabetes, metabolic syndrome: what should every pediatrician know? J Clin Res Pediatr Endocrinol. 2017; 9(Suppl 2):49–57.
Article29. Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991; 266:3008–11.
Article30. Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010; 7:251–64.
Article31. Lin WT, Huang HL, Huang MC, Chan TF, Ciou SY, Lee CY, et al. Effects on uric acid, body mass index and blood pressure in adolescents of consuming beverages sweetened with high-fructose corn syrup. Int J Obes (Lond). 2013; 37:532–9.
Article32. Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008; 300:924–32.
Article33. Madero M, Rodríguez Castellanos FE, Jalal D, VillalobosMartín M, Salazar J, Vazquez-Rangel A, et al. A pilot study on the impact of a low fructose diet and allopurinol on clinic blood pressure among overweight and prehypertensive subjects: a randomized placebo controlled trial. J Am Soc Hypertens. 2015; 9:837–44.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Four-Year Change of Metabolic Syndrome Incidence According to Serum Uric Acid
- Does Hyperuricemia Play a Causative Role in the Development and/or Aggravation of Renal, Cardiovascular and Metabolic Disease?
- Interrelationship of Uric Acid, Gout, and Metabolic Syndrome: Focus on Hypertension, Cardiovascular Disease, and Insulin Resistance
- Association between Serum Uric Acid and Metabolic Syndrome in Koreans
- The impact of uric acid and metabolic syndrome on the incidence of hypertention in a Korean population