Korean J Physiol Pharmacol.
2020 Jul;24(4):349-362. 10.4196/kjpp.2020.24.4.349.
Analysis of temperature-dependent abnormal bursting patterns of neurons in Aplysia
- Affiliations
-
- 1Department of Physics, Jeju National University, Jeju 63243, Korea
- 2Jeju Eastern Health Center, Jeju 63357, Korea
- 3Department of Anesthesiology and Pain Medicine, Daejeon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Daejeon 34943, Korea
- 4Laboratory for Behavioral Neural Circuitry and Physiology, Department of Anatomy, Brain Science and Engineering Institute, School of Medicine, Kyungpook National University, Daegu 41944, Korea
- KMID: 2503328
- DOI: http://doi.org/10.4196/kjpp.2020.24.4.349
Abstract
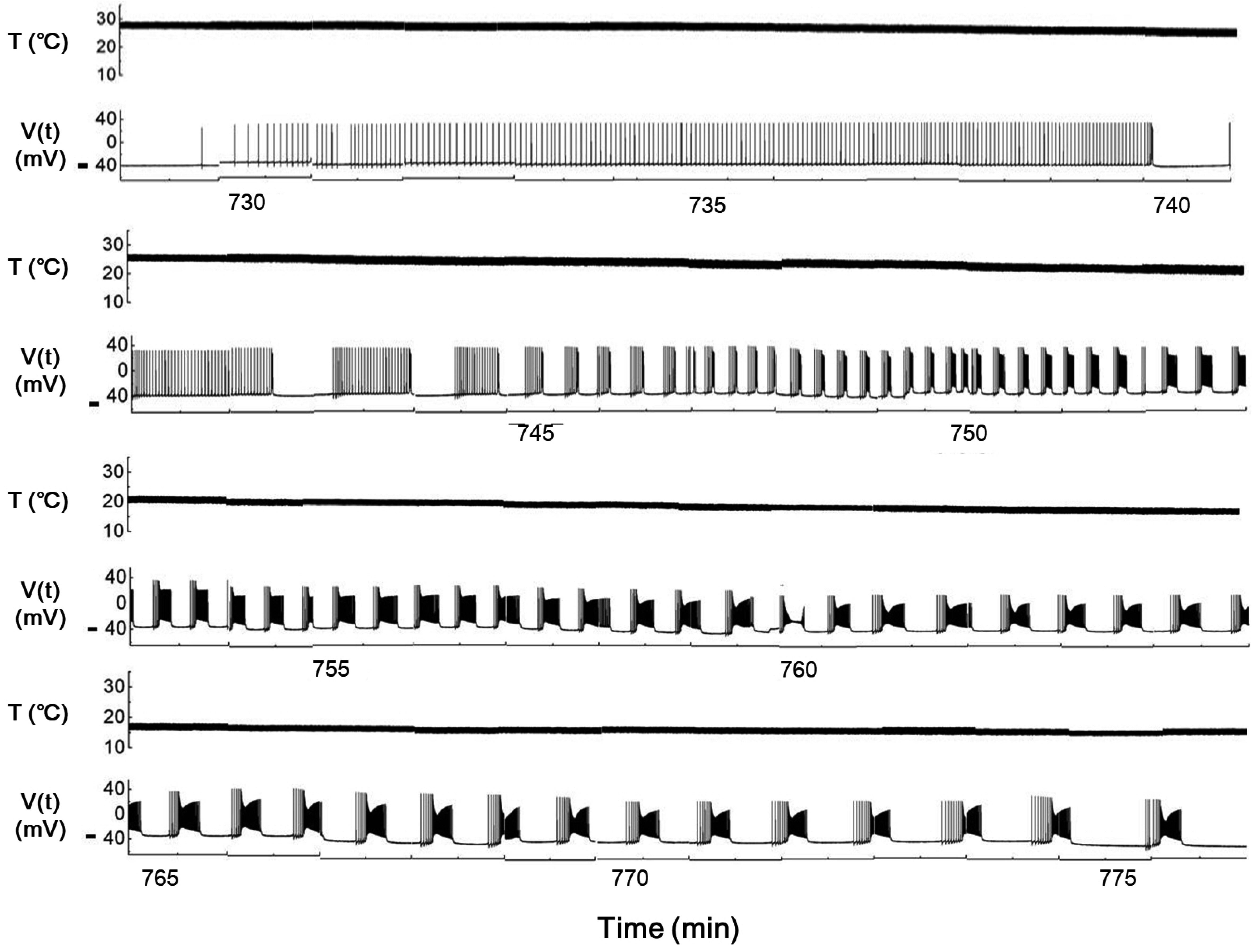
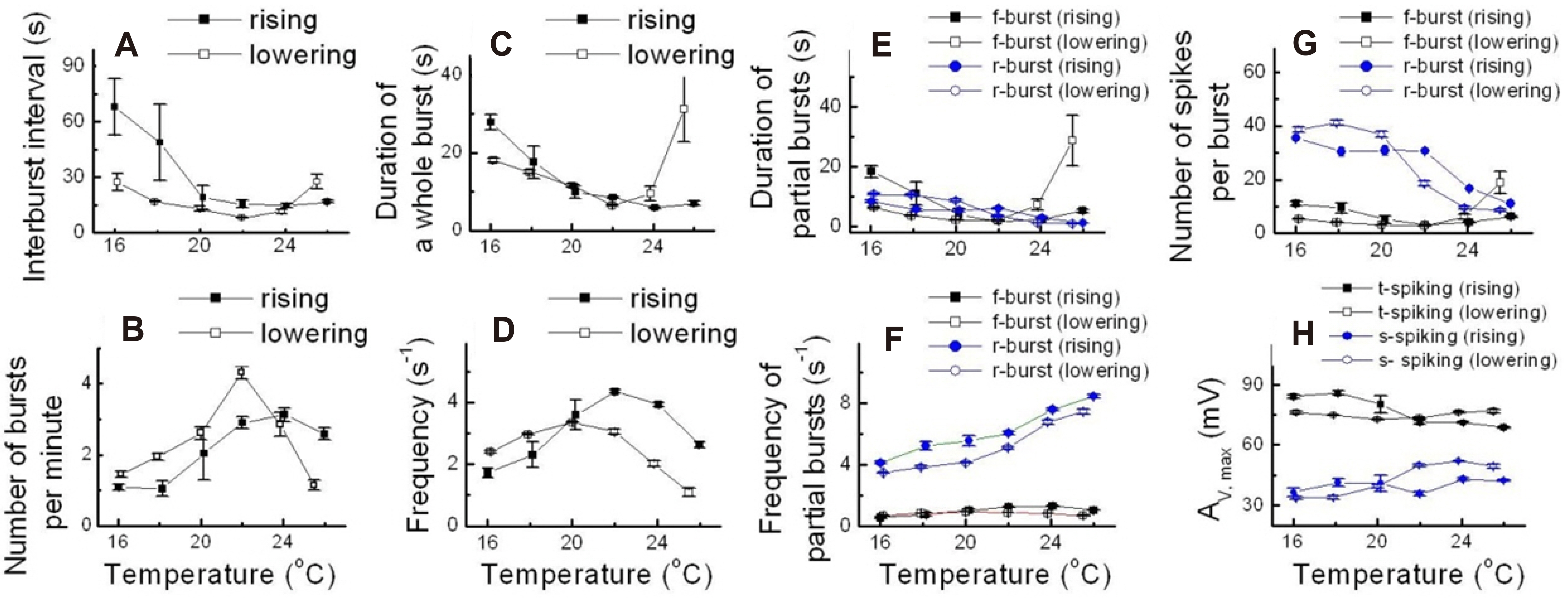
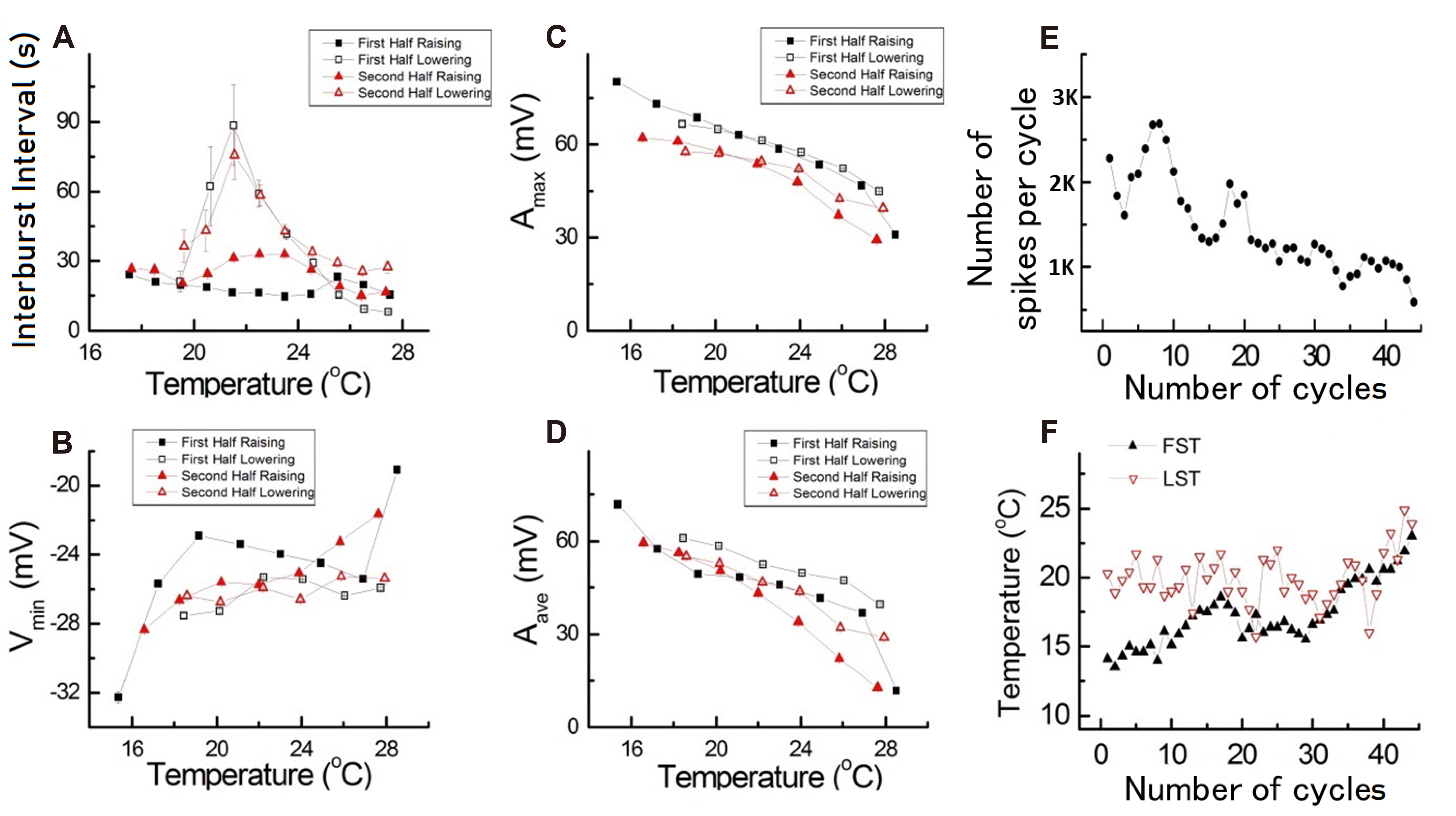
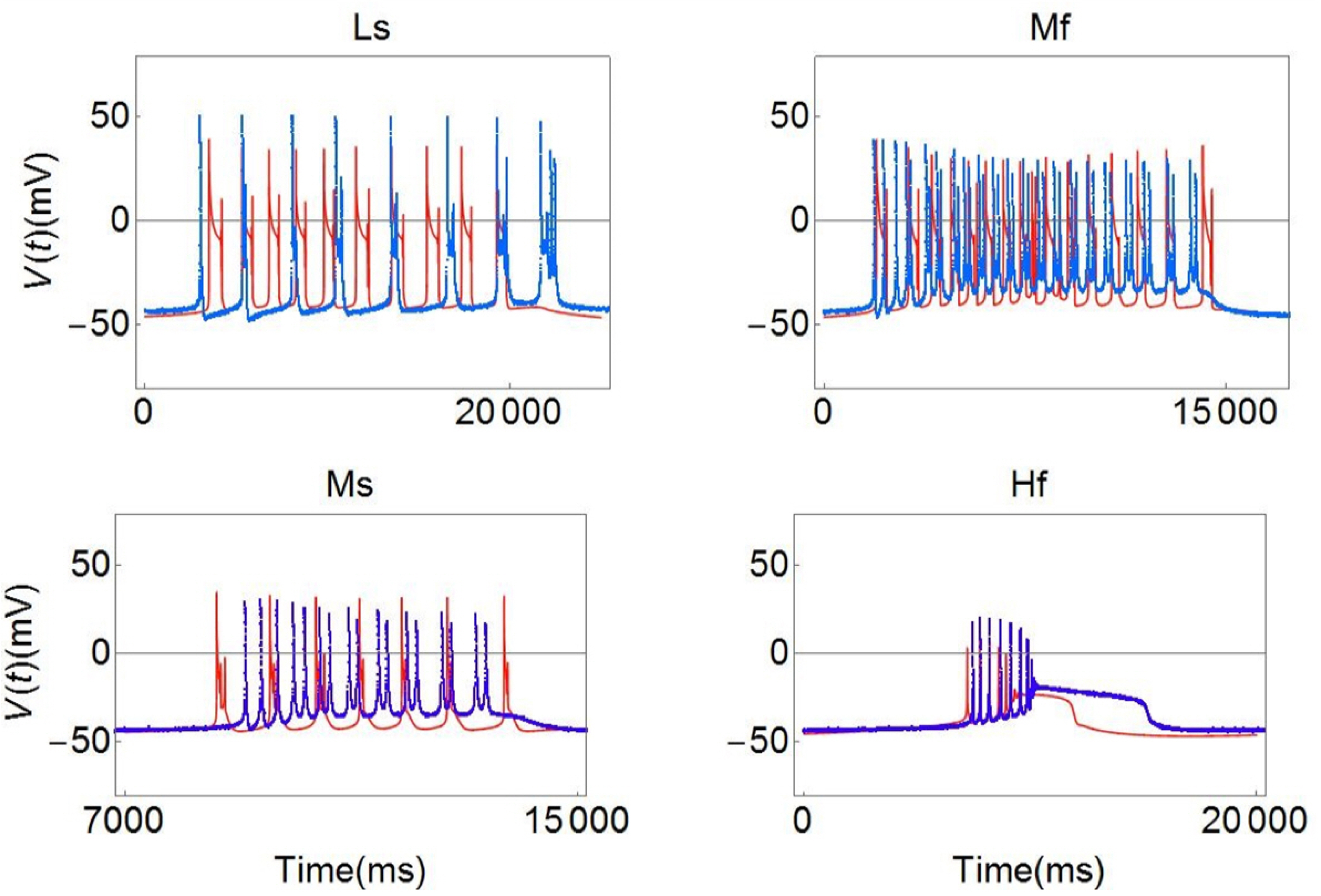
- Temperature affects the firing pattern and electrical activity of neurons in animals, eliciting diverse responses depending on neuronal cell type. However, the mechanisms underlying such diverse responses are not well understood. In the present study, we performed in vitro recording of abdominal ganglia cells of Aplysia juliana , and analyzed their burst firing patterns. We identified atypical bursting patterns dependent on temperature that were totally different from classical bursting patterns observed in R15 neurons of A. juliana . We classified these abnormal bursting patterns into type 1 and type 2; type 1 abnormal single bursts are composed of two kinds of spikes with a long interspike interval (ISI) followed by short ISI regular firing, while type 2 abnormal single bursts are composed of complex multiplets. To investigate the mechanism underlying the temperature dependence of abnormal bursting, we employed simulations using a modified Plant model and determined that the temperature dependence of type 2 abnormal bursting is related to temperaturedependent scaling factors and activation or inactivation of potassium or sodium channels.
Figure
Reference
-
1. Burrows M. 1989; Effects of temperature on a central synapse between identified motor neurons in the locust. J Comp Physiol A. 165:687–695. DOI: 10.1007/BF00611000. PMID: 2795499.2. Dalton JC, Hendrix DE. 1962; Effects of temperature on membrane potentials of lobster giant axon. Am J Physiol. 202:491–494. DOI: 10.1152/ajplegacy.1962.202.3.491. PMID: 13883255.
Article3. Frankenhaeuser B, Moore LE. 1963; The effect of temperature on the sodium and potassium permeability changes in myelinated nerve fibres of Xenopus laevis. J Physiol. 169:431–437. DOI: 10.1113/jphysiol.1963.sp007269. PMID: 14079679. PMCID: PMC1368766.4. Heitler WJ, Edwards DH. 1998; Effect of temperature on a voltage-sensitive electrical synapse in crayfish. J Exp Biol. 201(Pt 4):503–513. PMID: 9438826.
Article5. Hodgkin AL, Katz B. 1949; The effect of temperature on the electrical activity of the giant axon of the squid. J Physiol. 109:240–249. DOI: 10.1113/jphysiol.1949.sp004388. PMID: 15394322. PMCID: PMC1392577.
Article6. Kerkut GA, Ridge RM. 1962; The effect of temperature changes on the activity of the neurones of the snail Helix aspersa. Comp Biochem Physiol. 5:283–295. DOI: 10.1016/0010-406X(62)90057-9.7. Hyun NG, Hyun KH, Hyun KB, Han JH, Lee K, Kaang BK. 2011; A computational model of the temperature-dependent changes in firing patterns in Aplysia neurons. Korean J Physiol Pharmacol. 15:371–382. DOI: 10.4196/kjpp.2011.15.6.371. PMID: 22359475. PMCID: PMC3282225.8. Hyun NG, Hyun KH, Hyun KB, Lee K. 2014; Temperature-dependent bursting pattern analysis by modified Plant model. Mol Brain. 7:50. DOI: 10.1186/s13041-014-0050-5. PMID: 25051923. PMCID: PMC4223606.
Article9. Hyun NG, Hyun KH, Lee K, Kaang BK. 2012; Temperature dependence of action potential parameters in Aplysia neurons. Neurosignals. 20:252–264. DOI: 10.1159/000334960. PMID: 22286202.10. Lim CS, Chung DY, Kaang BK. 1997; Partial anatomical and physiological characterization and dissociated cell culture of the nervous system of the marine mollusc Aplysia kurodai. Mol Cells. 7:399–407.11. Lee SH, Lim CS, Park H, Lee JA, Han JH, Kim H, Cheang YH, Lee SH, Lee YS, Ko HG, Jang DH, Kim H, Miniaci MC, Bartsch D, Kim E, Bailey CH, Kandel ER, Kaang BK. 2007; Nuclear translocation of CAM-associated protein activates transcription for long-term facilitation in Aplysia. Cell. 129:801–812. DOI: 10.1016/j.cell.2007.03.041. PMID: 17512412.12. Plant RE. 1981; Bifurcation and resonance in a model for bursting nerve cells. J Math Biol. 11:15–32. DOI: 10.1007/BF00275821. PMID: 7252375.
Article13. Plant RE, Kim M. 1976; Mathematical description of a bursting pacemaker neuron by a modification of the Hodgkin-Huxley equations. Biophys J. 16:227–244. DOI: 10.1016/S0006-3495(76)85683-4.
Article14. Finke C, Freund JA, Rosa E Jr, Braun HA, Feudel U. 2010; On the role of subthreshold currents in the Huber-Braun cold receptor model. Chaos. 20:045107. DOI: 10.1063/1.3527989. PMID: 21198119.
Article15. Rinzel J, Lee YS. 1987; Dissection of a model for neuronal parabolic bursting. J Math Biol. 25:653–675. DOI: 10.1007/BF00275501. PMID: 3437231.
Article16. Picardo MC, Weragalaarachchi KT, Akins VT, Del Negro CA. 2013; Physiological and morphological properties of Dbx1-derived respiratory neurons in the pre-Botzinger complex of neonatal mice. J Physiol. 591:2687–2703. DOI: 10.1113/jphysiol.2012.250118. PMID: 23459755. PMCID: PMC3678050.17. Pace RW, Mackay DD, Feldman JL, Del Negro CA. 2007; Inspiratory bursts in the preBötzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J Physiol. 582(Pt 1):113–125. DOI: 10.1113/jphysiol.2007.133660. PMID: 17446214. PMCID: PMC2075310.
Article18. Paton JF, Abdala AP, Koizumi H, Smith JC, St-John WM. 2006; Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci. 9:311–313. DOI: 10.1038/nn1650. PMID: 16474390.
Article19. Rubin JE, Hayes JA, Mendenhall JL, Del Negro CA. 2009; Calcium-activated nonspecific cation current and synaptic depression promote network-dependent burst oscillations. Proc Natl Acad Sci U S A. 106:2939–2944. DOI: 10.1073/pnas.0808776106. PMID: 19196976. PMCID: PMC2636730.
Article20. Hou N, Armstrong GA, Chakraborty-Chatterjee M, Sokolowski MB, Robertson RM. 2014; Na+-K+-ATPase trafficking induced by heat shock pretreatment correlates with increased resistance to anoxia in locusts. J Neurophysiol. 112:814–823. DOI: 10.1152/jn.00201.2014. PMID: 24848469. PMCID: PMC4122745.21. Money TG, Rodgers CI, McGregor SM, Robertson RM. 2009; Loss of potassium homeostasis underlies hyperthermic conduction failure in control and preconditioned locusts. J Neurophysiol. 102:285–293. DOI: 10.1152/jn.91174.2008. PMID: 19386751.
Article22. Pulver SR, Griffith LC. 2010; Spike integration and cellular memory in a rhythmic network from Na+/K+ pump current dynamics. Nat Neurosci. 13:53–59. DOI: 10.1038/nn.2444. PMID: 19966842. PMCID: PMC2839136.23. Money TG, Anstey ML, Robertson RM. 2005; Heat stress-mediated plasticity in a locust looming-sensitive visual interneuron. J Neurophysiol. 93:1908–1919. DOI: 10.1152/jn.00908.2004. PMID: 15563551.
Article24. Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honoré E. 2000; TREK-1 is a heat-activated background K+ channel. EMBO J. 19:2483–2491. DOI: 10.1093/emboj/19.11.2483. PMID: 10835347. PMCID: PMC212769.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Computational Model of the Temperature-dependent Changes in Firing Patterns in Aplysia Neurons
- The Effect of Heat on the Spiking Patterns of the Cells in Aplysia
- Specific Expression of Aplysia Phosphodiesterase 4 in Bag Cells Revealed by in situ Hybridization Analysis
- Neuronal RNA granule contains ApCPEB1, a novel cytoplasmic polyadenylation element binding protein, in Aplysia sensory neuron
- Roles of Ca2+ activated K+ conductances on spontaneous firing patterns of isolated rat medial vestibular nucleus neurons