J Rhinol.
2020 May;27(1):34-40. 10.18787/jr.2019.00301.
Effect of Tobacco-specific Nitrosamines on MUC5AC Expression in Human Airway Epithelial Cells
- Affiliations
-
- 1Department of Medical Science, College of Medicine, Graduate School of Yeungnam University, Daegu, Korea
- 2Department of Otorhinolaryngology-Head and Neck Surgery, College of Medicine, Yeungnam University, Daegu, Korea
- 3Regional Center for Respiratory Diseases, Yeungnam University Medical Center, Daegu, Korea
- KMID: 2502792
- DOI: http://doi.org/10.18787/jr.2019.00301
Abstract
- Background and Objectives
Nicotine is oxidized into tobacco-specific nitrosamines (TSNAs; NAB, NAT, NNN, NNAL, NNK) at high temperature and high pressure. TSNAs are associated with airway diseases characterized by mucus hypersecretion as a major pathophysiologic phenomenon. The aim of study is to investigate the effect of TSNAs on mucin overexpression and its molecular mechanism in human airway epithelial cells. Materials and Method: The cytotoxicity of TSNAs was evaluated using EX-Cytox and inverted microscopy. The mRNA and protein levels of MUC5AC and MUC5B were measured using real-time PCR and ELISA.
Results
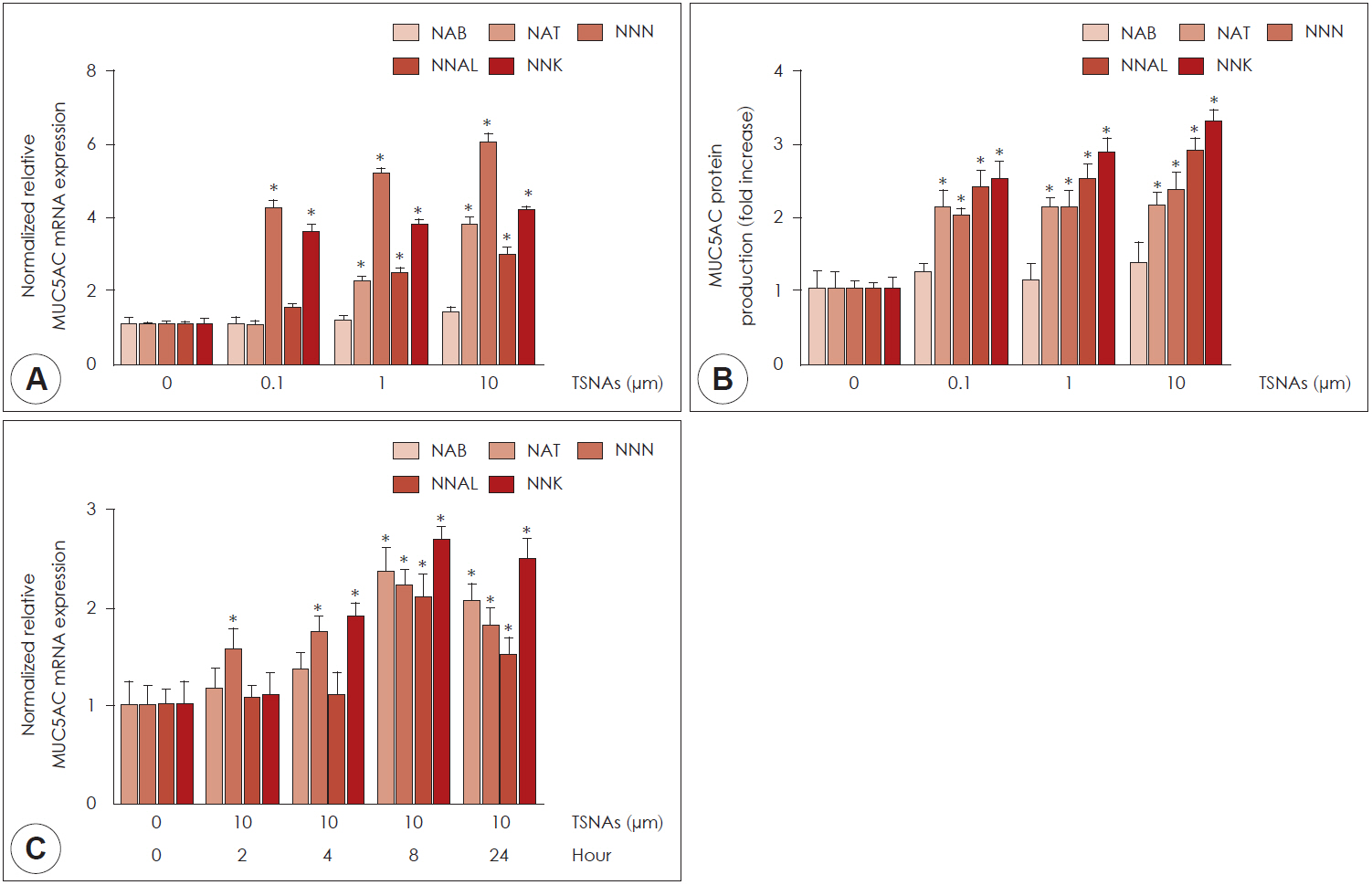
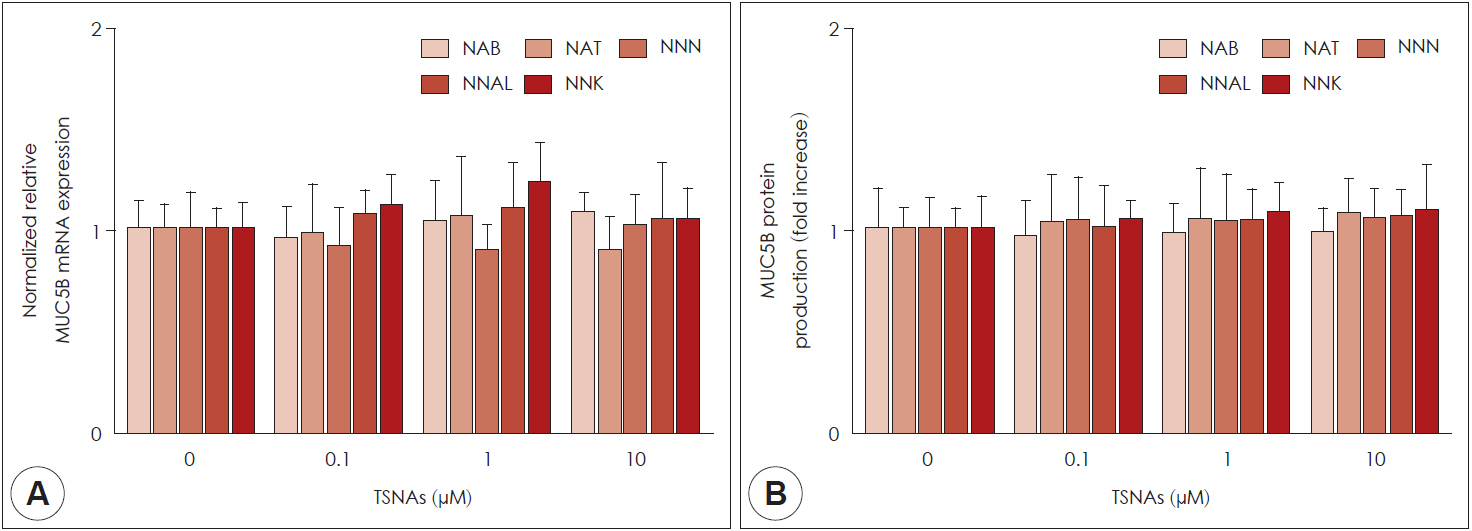
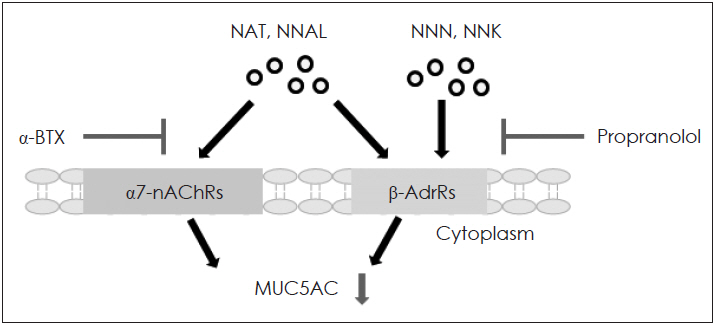
NAB, NNN, NNAL, and NNK did not affect cell viability. NAT did not affect cell viability up to a concentration of 100 μM in human airway epithelial cells. NAT, NNN, NNAL, and NNK significantly induced MUC5AC expression, but not MUC5B expression. NAB did not affect the expression of MUC5AC and MUC5B. Propranolol (a β-adrenergic receptor antagonist) inhibited NAT, NNN, NNAL, and NNK-induced MUC5AC expression, whereas α-bungarotoxin (an α7-nicotinic acetylcholine receptor antagonist) only inhibited NNN- and NNK-induced MUC5AC expression.
Conclusion
These results suggested that NAT, NNN, NNAL, and NNK induce MUC5AC expression through β-adrenergic receptor and/or α7-nicotinic acetylcholine receptor in human airway epithelial cells, which may be involved in mucus hypersecretion in inflammatory airway diseases.
Keyword
Figure
Reference
-
1. Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010; 10:231.2. Clapp PW, Jaspers I. Electronic Cigarettes: Their constituents and potential links to asthma. Curr Allergy Asthm R. 2017; 17(11):79.3. Edwards SH, Rossiter LM, Taylor KM, Holman MR, Zhang L, Ding YS, et al. Tobacco-specific nitrosamines in the tobacco and mainstream smoke of U.S. commercial cigarettes. Chem Res Toxicol. 2017; 30(2):540–51.4. Lu J, Zhang L, Lewis RS, Bovet L, Goepfert S, Jack AM, et al. Expression of a constitutively active nitrate reductase variant in tobacco reduces tobacco-specific nitrosamine accumulation in cured leaves and cigarette smoke. Plant Biotechnol J. 2016; 14(7):1500–10.5. Yalcin E, de la Monte S. Tobacco nitrosamines as culprits in disease: mechanisms reviewed. J Physiol Biochem. 2016; 72(1):107–20.6. Gupta AK, Tulsyan S, Bharadwaj M, Mehrotra R. Grass roots approach to control levels of carcinogenic nitrosamines, NNN and NNK in smokeless tobacco products. Food Chem Toxicol. 2019; 124:359–66.7. Hauber HP, Foley SC, Hamid Q. Mucin overproduction in chronic inflammatory lung disease. Can Respir J. 2006; 13(6):327–35.8. Daviskas E, Anderson SD, Shaw J, Eberl S, Seale JP, Yang IA, et al. Mucociliary clearance in patients with chronic asthma: Effects of beta(2) agonists. Respirology. 2005; 10(4):426–35.9. Ma J, Rubin BK, Voynow JA. Mucins, mucus, and goblet Cells. Chest. 2018; 154(1):169–76.10. Park NK, Choi YS, Lee JH, Kim HS, Kim JK, Ahn JH, et al. Effect of udenafil on MUC5B expression in human airway epithelial cells. Korean J Otorhinolaryngol-Head Neck Surg. 2013; 56(8):501–5.11. Lee JG, Moon HJ, Kim SS, Kim CW, Yoon JH. Expression and regulation of MUC8 & MUC5AC by various cytokines in normal human nasal epithelial cells. Korean J Otorhinolaryngol-Head Neck Surg. 2001; 44(6):600–5.12. Gray T, Nettesheim P, Loftin C, Koo JS, Bonner J, Peddada S, et al. Interleukin-1beta-induced mucin production in human airway epithelium is mediated by cyclooxygenase-2, prostaglandin E2 receptors, and cyclic AMP-protein kinase A signaling. Mol Pharmacol. 2004; 66(2):337–46.13. Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003; 278(19):17036–43.14. Bautista MV, Chen YJ, Ivanova VS, Rahimi MK, Watson AM, Rose MC. IL-8 Regulates Mucin Gene Expression at the Posttranscriptional Level in Lung Epithelial Cells. J Immunol. 2009; 183(3):2159–66.15. Song SY, Bae CH, Choi YS, Kim YD. Cadmium induces mucin 8 expression via Toll-like receptor 4-mediated extracellular signal related kinase 1/2 and p38 mitogen-activated protein kinase in human airway epithelial cells. Int Forum Allergy Rhinol. 2016; 6(6):638–45.16. Na HG, Kim YD, Choi YS, Bae CH, Song SY. Diesel exhaust particles elevate MUC5AC and MUC5B expression via the TLR4-mediated activation of ERK1/2, p38 MAPK, and NF-kappaB signaling pathways in human airway epithelial cells. Biochem Biophys Res Commun. 2019; 512(1):53–9.17. Deshmukh HS, Shaver C, Case LM, Dietsch M, Wesselkamper SC, Hardie WD, et al. Acrolein-activated matrix metalloproteinase 9 contributes to persistent mucin production. Am J Respir Cell Mol Biol. 2008; 38(4):446–54.18. Meo SA, Al Asiri SA. Effects of electronic cigarette smoking on human health. Eur Rev Med Pharmacol Sci. 2014; 18(21):3315–9.19. Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine Tob Res. 2006; 8(2):309–13.20. Moir D, Rickert WS, Levasseur G, Larose Y, Maertens R, White P, et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol. 2008; 21(2):494–502.21. Carson JL, Brighton LE, Jaspers I. Phenotypic modification of human airway epithelial cells in air-liquid interface culture induced by exposure to the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Ultrastruct Pathol. 2015; 39(2):104–9.22. Ridley C, Thornton DJ. Mucins: the frontline defence of the lung. Biochem Soc Trans. 2018; 46(5):1099–106.23. Bonser LR, Erle DJ. Airway mucus and asthma: The Role of MUC5AC and MUC5B. J Clin Med. 2017; 6(12):112.24. Groneberg DA, Eynott PR, Oates T, Lim S, Wu R, Carlstedt I, et al. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respir Med. 2002; 96(2):81–6.25. Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature. 2014; 505(7483):412–6.26. Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, et al. Airway mucin concentration as a marker of chronic bronchitis. New Engl J Med. 2017; 377(10):911–22.27. Wu J. Understanding of nicotinic acetylcholine receptors. Acta Pharmacol Sin. 2009; 30(6):653–5.28. Wu CH, Lee CH, Ho YS. Nicotinic Acetylcholine Receptor-Based Blockade: Applications of Molecular Targets for Cancer Therapy. Clin Cancer Res. 2011; 17(11):3533–41.29. Wang Y, Pereira EF, Maus AD, Ostlie NS, Navaneetham D, Lei S, et al. Human bronchial epithelial and endothelial cells express alpha7 nicotinic acetylcholine receptors. Mol Pharmacol. 2001; 60(6):1201–9.30. Shin VY, Jin HC, Ng EKO, Yu J, Leung WK, Cho CH, et al. Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induce cyclooxygenase-2 activity in human gastric cancer cells: Involvement of nicotinic acetylcholine receptor (nAChR) and beta-adrenergic receptor signaling pathways. Toxicol Appl Pharm. 2008; 233(2):254–61.31. Zhou Y, Zhang Y, Guo Y, Zhang Y, Xu M, He B. beta2-Adrenoceptor involved in smoking-induced airway mucus hypersecretion through beta-arrestin-dependent signaling. PLoS One. 2014; 9(6):e97788.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Multi-Walled Carbon Nanotubes on MUC5AC and MUC5B Expression in Airway Epithelial Cells
- Changes in Mucin Production in Human Airway Epithelial Cells After Exposure to Electronic Cigarette Vapor With or Without Nicotine
- Effect of High-Insulin on MUC4, MUC5AC, and MUC5B Expression in Airway Epithelial Cells
- Inhibitory Effects of Protopanaxadiol on Lipopolysaccharide-Induced Reactive Oxygen Species Production and MUC5AC Expression in Human Airway Epithelial Cells
- Eriodictyol Inhibits the Production and Gene Expression of MUC5AC Mucin via the IκBα-NF-κB p65 Signaling Pathway in Airway Epithelial Cells