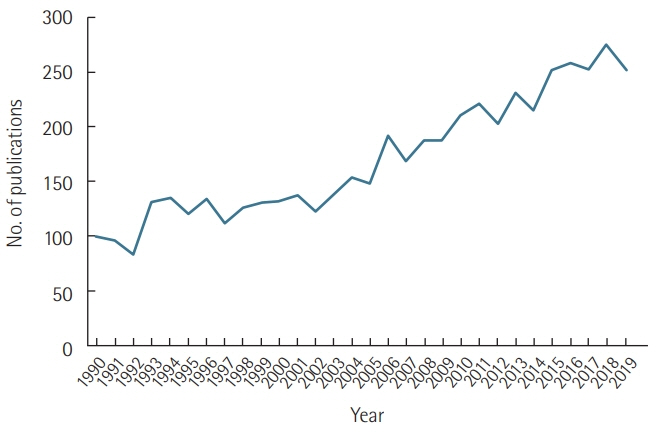
Acute Crit Care.
2020 May;35(2):57-66. 10.4266/acc.2020.00248.
Sepsis-induced cardiac dysfunction: a review of pathophysiology
- Affiliations
-
- 1Chonnam National University Graduate School, Gwangju, Korea
- 2Department of Pediatrics, Chonnam National University Children’s Hospital, Gwangju, Korea
- 3Department of Pediatrics, Chonnam National University Children’s Hospital and Medical School, Gwangju, Korea
- 4Department of Thoracic and Cardiovascular Surgery, Chonnam National University Hospital and Medical School, Gwangju, Korea
- KMID: 2502335
- DOI: http://doi.org/10.4266/acc.2020.00248
Abstract
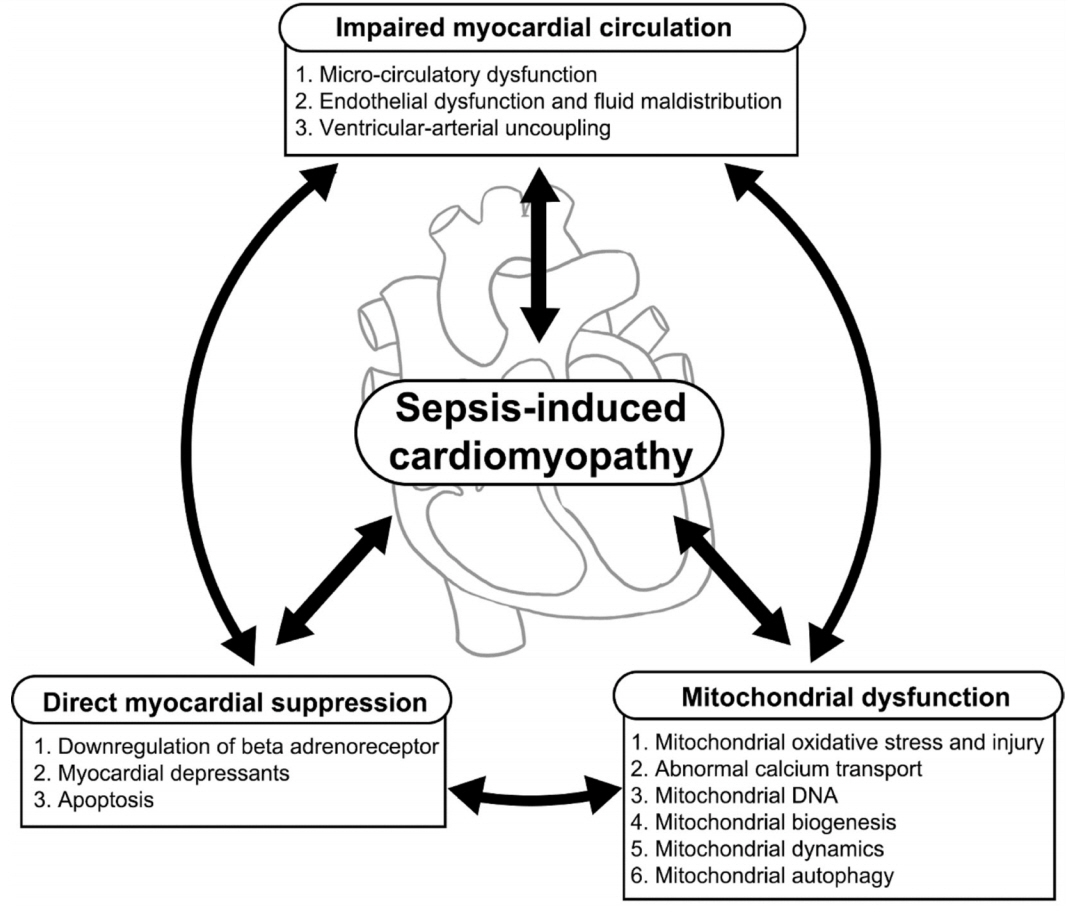
- It is well known that cardiac dysfunction in sepsis is associated with significantly increased mortality. The pathophysiology of sepsis-induced cardiac dysfunction can be summarized as involving impaired myocardial circulation, direct myocardial depression, and mitochondrial dysfunction. Impaired blood flow to the myocardium is associated with microvascular dysfunction, impaired endothelium, and ventriculo-arterial uncoupling. The mechanisms behind direct myocardial depression consist of downregulation of β-adrenoceptors and several myocardial suppressants (such as cytokine and nitric oxide). Recent research has highlighted that mitochondrial dysfunction, which results in energy depletion, is a major factor in sepsis-induced cardiac dysfunction. Therefore, the authors summarize the pathophysiological process of cardiac dysfunction in sepsis based on the results of recent studies.
Keyword
Figure
Reference
-
1. Vieillard-Baron A. Septic cardiomyopathy. Ann Intensive Care. 2011; 1:6.
Article2. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003; 348:138–50.
Article3. Geri G, Vignon P, Aubry A, Fedou AL, Charron C, Silva S, et al. Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: a post hoc analysis. Intensive Care Med. 2019; 45:657–67.
Article4. Fernandes CJ Jr, de Assuncao MS. Myocardial dysfunction in sepsis: a large, unsolved puzzle. Crit Care Res Pract. 2012; 2012:896430.5. L’Heureux M, Sternberg M, Brath L, Turlington J, Kashiouris MG. Sepsis-induced cardiomyopathy: a comprehensive review. Curr Cardiol Rep. 2020; 22:35.
Article6. Pulido JN, Afessa B, Masaki M, Yuasa T, Gillespie S, Herasevich V, et al. Clinical spectrum, frequency, and significance of myocardial dysfunction in severe sepsis and septic shock. Mayo Clin Proc. 2012; 87:620–8.
Article7. Muller-Werdan U, Buerke M, Ebelt H, Heinroth KM, Herklotz A, Loppnow H, et al. Septic cardiomyopathy: a not yet discovered cardiomyopathy? Exp Clin Cardiol. 2006; 11:226–36.8. Hoesel LM, Niederbichler AD, Ward PA. Complement-related molecular events in sepsis leading to heart failure. Mol Immunol. 2007; 44:95–102.
Article9. Parker MM, Suffredini AF, Natanson C, Ognibene FP, Shelhamer JH, Parrillo JE. Responses of left ventricular function in survivors and nonsurvivors of septic shock. J Crit Care. 1989; 4:19–25.
Article10. Poelaert J, Declerck C, Vogelaers D, Colardyn F, Visser CA. Left ventricular systolic and diastolic function in septic shock. Intensive Care Med. 1997; 23:553–60.
Article11. Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984; 100:483–90.
Article12. Parker MM, McCarthy KE, Ognibene FP, Parrillo JE. Right ventricular dysfunction and dilatation, similar to left ventricular changes, characterize the cardiac depression of septic shock in humans. Chest. 1990; 97:126–31.
Article13. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006; 124:783–801.
Article14. Raeburn CD, Sheppard F, Barsness KA, Arya J, Harken AH. Cytokines for surgeons. Am J Surg. 2002; 183:268–73.
Article15. Levy RJ, Piel DA, Acton PD, Zhou R, Ferrari VA, Karp JS, et al. Evidence of myocardial hibernation in the septic heart. Crit Care Med. 2005; 33:2752–6.
Article16. Antonucci E, Fiaccadori E, Donadello K, Taccone FS, Franchi F, Scolletta S. Myocardial depression in sepsis: from pathogenesis to clinical manifestations and treatment. J Crit Care. 2014; 29:500–11.
Article17. Kakihana Y, Ito T, Nakahara M, Yamaguchi K, Yasuda T. Sepsis-induced myocardial dysfunction: pathophysiology and management. J Intensive Care. 2016; 4:22.
Article18. Kumar A, Krieger A, Symeoneides S, Kumar A, Parrillo JE. Myocardial dysfunction in septic shock. Part II. Role of cytokines and nitric oxide. J Cardiothorac Vasc Anesth. 2001; 15:485–511.
Article19. Pathan N, Hemingway CA, Alizadeh AA, Stephens AC, Boldrick JC, Oragui EE, et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004; 363:203–9.
Article20. Loppnow H, Werdan K, Reuter G, Flad HD. The interleukin-1 and interleukin-1 converting enzyme families in the cardiovascular system. Eur Cytokine Netw. 1998; 9:675–80.21. Kelly RA, Balligand JL, Smith TW. Nitric oxide and cardiac function. Circ Res. 1996; 79:363–80.
Article22. Francis SE, Holden H, Holt CM, Duff GW. Interleukin-1 in myocardium and coronary arteries of patients with dilated cardiomyopathy. J Mol Cell Cardiol. 1998; 30:215–23.
Article23. Opal SM, Fisher CJ Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo- controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med. 1997; 25:1115–24.24. Müller-Werdan U, Werdan K. Immune modulation by catecholamines: a potential mechanism of cytokine release in heart failure? Herz. 2000; 25:271–3.25. Geppert A, Steiner A, Zorn G, Delle-Karth G, Koreny M, Haumer M, et al. Multiple organ failure in patients with cardiogenic shock is associated with high plasma levels of interleukin-6. Crit Care Med. 2002; 30:1987–94.
Article26. Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med. 2007; 35:1599–608.
Article27. Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care. 2009; 15:392–7.
Article28. Crouser ED, Julian MW, Huff JE, Joshi MS, Bauer JA, Gadd ME, et al. Abnormal permeability of inner and outer mitochondrial membranes contributes independently to mitochondrial dysfunction in the liver during acute endotoxemia. Crit Care Med. 2004; 32:478–88.
Article29. Memiş D, Karamanlioğlu B, Turan A, Koyuncu O, Pamukçu Z. Effects of lornoxicam on the physiology of severe sepsis. Crit Care. 2004; 8:R474–82.30. Konrad D, Oldner A, Rossi P, Wanecek M, Rudehill A, Weitzberg E. Differentiated and dose-related cardiovascular effects of a dual endothelin receptor antagonist in endotoxin shock. Crit Care Med. 2004; 32:1192–9.
Article31. Raeburn CD, Calkins CM, Zimmerman MA, Song Y, Ao L, Banerjee A, et al. ICAM-1 and VCAM-1 mediate endotoxemic myocardial dysfunction independent of neutrophil accumulation. Am J Physiol Regul Integr Comp Physiol. 2002; 283:R477–86.32. Raeburn CD, Calkins CM, Zimmerman MA, Song Y, Ao L, Banerjee A, et al. Vascular cell adhesion molecule: 1 expression is obligatory for endotoxin-induced myocardial neutrophil accumulation and contractile dysfunction. Surgery. 2001; 130:319–25.33. Niederbichler AD, Hoesel LM, Westfall MV, Gao H, Ipaktchi KR, Sun L, et al. An essential role for complement C5a in the pathogenesis of septic cardiac dysfunction. J Exp Med. 2006; 203:53–61.
Article34. Alhamdi Y, Abrams ST, Cheng Z, Jing S, Su D, Liu Z, et al. Circulating histones are major mediators of cardiac injury in patients with sepsis. Crit Care Med. 2015; 43:2094–103.
Article35. VanPatten S, Al-Abed Y. High mobility group box-1 (HMGb1):current wisdom and advancement as a potential drug target. J Med Chem. 2018; 61:5093–107.36. Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: myocardial depression in sepsis and septic shock. Crit Care. 2002; 6:500–8.37. Witthaut R, Busch C, Fraunberger P, Walli A, Seidel D, Pilz G, et al. Plasma atrial natriuretic peptide and brain natriuretic peptide are increased in septic shock: impact of interleukin-6 and sepsis-associated left ventricular dysfunction. Intensive Care Med. 2003; 29:1696–702.
Article38. Grandel U, Hopf M, Buerke M, Hattar K, Heep M, Fink L, et al. Mechanisms of cardiac depression caused by lipoteichoic acids from Staphylococcus aureus in isolated rat hearts. Circulation. 2005; 112:691–8.39. Jang DH, Greenwood JC, Spyres MB, Eckmann DM. Measurement of mitochondrial respiration and motility in acute care: sepsis, trauma, and poisoning. J Intensive Care Med. 2017; 32:86–94.40. Carré JE, Singer M. Cellular energetic metabolism in sepsis: the need for a systems approach. Biochim Biophys Acta. 2008; 1777:763–71.
Article41. Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002; 360:219–23.
Article42. Potz BA, Sellke FW, Abid MR. Endothelial ROS and impaired myocardial oxygen consumption in sepsis-induced cardiac dysfunction. J Intensive Crit Care. 2016; 2:20.43. Larsen FJ, Schiffer TA, Weitzberg E, Lundberg JO. Regulation of mitochondrial function and energetics by reactive nitrogen oxides. Free Radic Biol Med. 2012; 53:1919–28.
Article44. Crouser ED. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion. 2004; 4:729–41.
Article45. Kayali R, Aydin S, Cakatay U. Effect of gender on main clinical chemistry parameters in aged rats. Curr Aging Sci. 2009; 2:67–71.
Article46. Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011; 107:57–64.
Article47. Lowes DA, Webster NR, Murphy MP, Galley HF. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br J Anaesth. 2013; 110:472–80.
Article48. Bernardi P, Di Lisa F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J Mol Cell Cardiol. 2015; 78:100–6.
Article49. Duncan DJ, Yang Z, Hopkins PM, Steele DS, Harrison SM. TNFalpha and IL-1beta increase Ca2+ leak from the sarcoplasmic reticulum and susceptibility to arrhythmia in rat ventricular myocytes. Cell Calcium. 2010; 47:378–86.50. Hassoun SM, Marechal X, Montaigne D, Bouazza Y, Decoster B, Lancel S, et al. Prevention of endotoxin-induced sarcoplasmic reticulum calcium leak improves mitochondrial and myocardial dysfunction. Crit Care Med. 2008; 36:2590–6.
Article51. Shanmuganathan S, Hausenloy DJ, Duchen MR, Yellon DM. Mitochondrial permeability transition pore as a target for cardioprotection in the human heart. Am J Physiol Heart Circ Physiol. 2005; 289:H237–42.
Article52. Smeding L, Plötz FB, Groeneveld AB, Kneyber MC. Structural changes of the heart during severe sepsis or septic shock. Shock. 2012; 37:449–56.
Article53. Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992; 257:387–9.
Article54. Wu LL, Ji Y, Dong LW, Liu MS. Calcium uptake by sarcoplasmic reticulum is impaired during the hypodynamic phase of sepsis in the rat heart. Shock. 2001; 15:49–55.
Article55. Galluzzi L, Kepp O, Trojel-Hansen C, Kroemer G. Mitochondrial control of cellular life, stress, and death. Circ Res. 2012; 111:1198–207.
Article56. Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012; 148:1145–59.
Article57. Harrington JS, Choi AM, Nakahira K. Mitochondrial DNA in Sepsis. Curr Opin Crit Care. 2017; 23:284–90.
Article58. Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011; 12:222–30.
Article59. Nakahira K, Kyung SY, Rogers AJ, Gazourian L, Youn S, Massaro AF, et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 2013; 10:e1001577.
Article60. Bhagirath VC, Dwivedi DJ, Liaw PC. Comparison of the proinflammatory and procoagulant properties of nuclear, mitochondrial, and bacterial DNA. Shock. 2015; 44:265–71.
Article61. Sánchez-Villamil JP, D’Annunzio V, Finocchietto P, Holod S, Rebagliati I, Pérez H, et al. Cardiac-specific overexpression of thioredoxin 1 attenuates mitochondrial and myocardial dysfunction in septic mice. Int J Biochem Cell Biol. 2016; 81(Pt B):323–34.
Article62. Liesa M, Palacín M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009; 89:799–845.
Article63. Zhan M, Brooks C, Liu F, Sun L, Dong Z. Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int. 2013; 83:568–81.
Article64. Wu Y, Yao YM, Lu ZQ. Mitochondrial quality control mechanisms as potential therapeutic targets in sepsis-induced multiple organ failure. J Mol Med (Berl). 2019; 97:451–62.
Article65. Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014; 5:66–72.
Article66. Gunst J, Derese I, Aertgeerts A, Ververs EJ, Wauters A, Van den Berghe G, et al. Insufficient autophagy contributes to mitochondrial dysfunction, organ failure, and adverse outcome in an animal model of critical illness. Crit Care Med. 2013; 41:182–94.
Article67. Rocha M, Herance R, Rovira S, Hernández-Mijares A, Victor VM. Mitochondrial dysfunction and antioxidant therapy in sepsis. Infect Disord Drug Targets. 2012; 12:161–78.
Article