J Korean Neurosurg Soc.
2020 May;63(3):327-337. 10.3340/jkns.2020.0018.
Junctional Neural Tube Defect
- Affiliations
-
- 1Department of Clinical Medicine, Faculty of Medicine and Health Sciences, Macquarie University, Sydney, Australia
- 2Department of Neurosurgery, Macquarie University Hospital, Sydney, Australia
- 3Department of Paediatric Neurosurgery, Sydney Children’s Hospital Randwick, Sydney, Australia
- 4Department of Paediatric Neurosurgery, Great Ormond Street Hospital for Children, NHS Trust, London, UK
- 5Department of Paediatric Neurosurgery, University of California, Davis, CA, USA
- KMID: 2501719
- DOI: http://doi.org/10.3340/jkns.2020.0018
Abstract
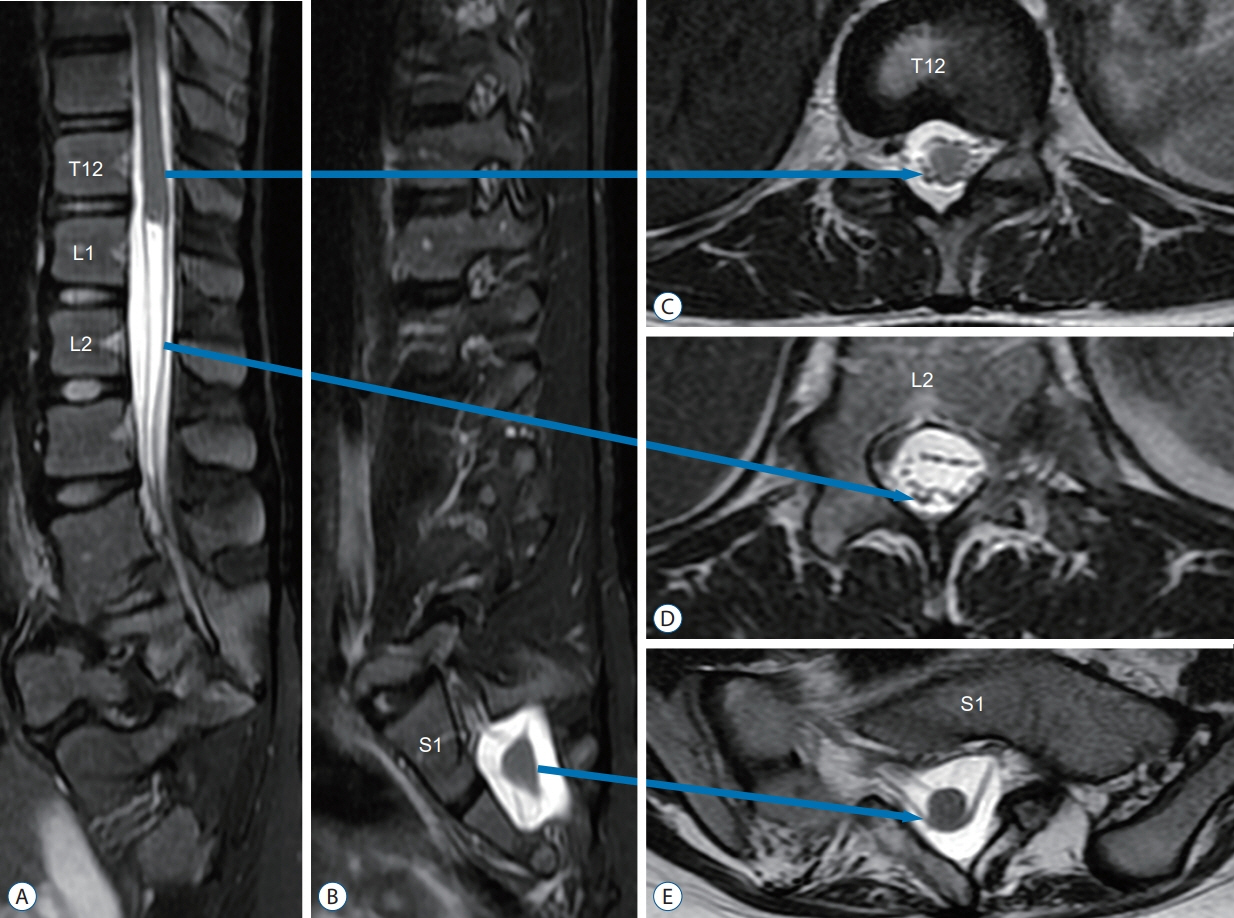
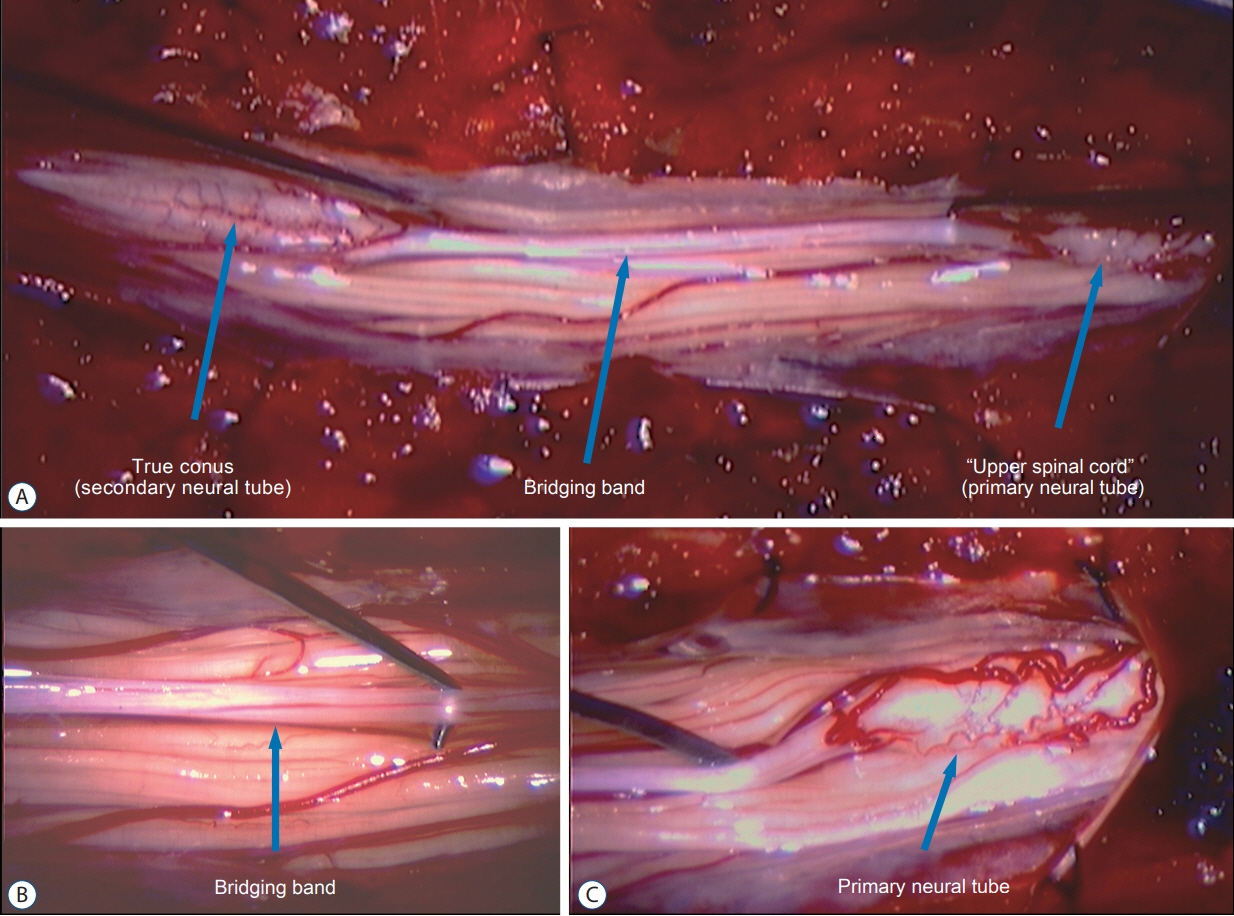
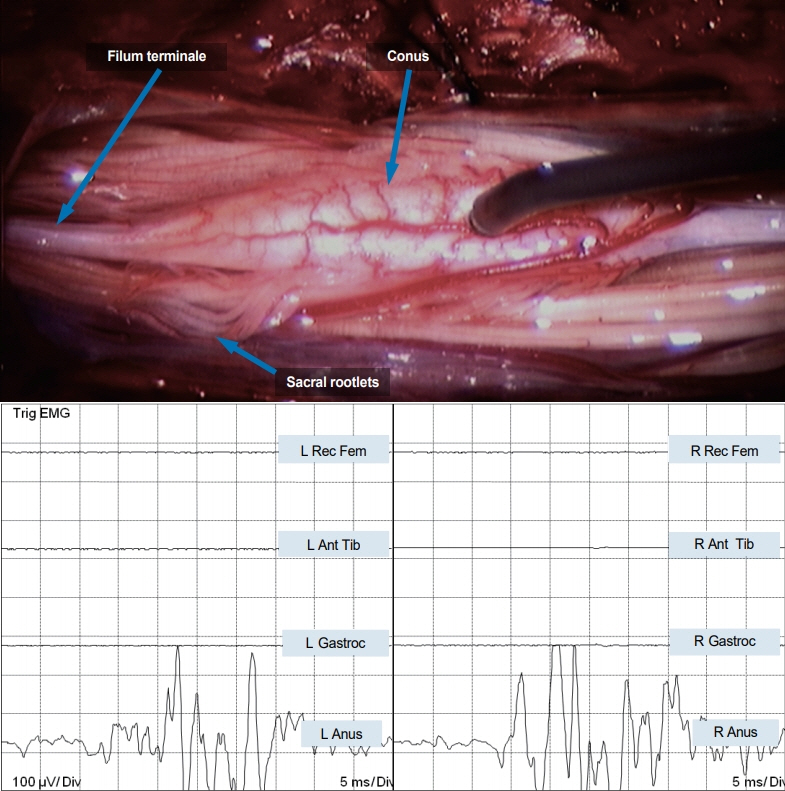
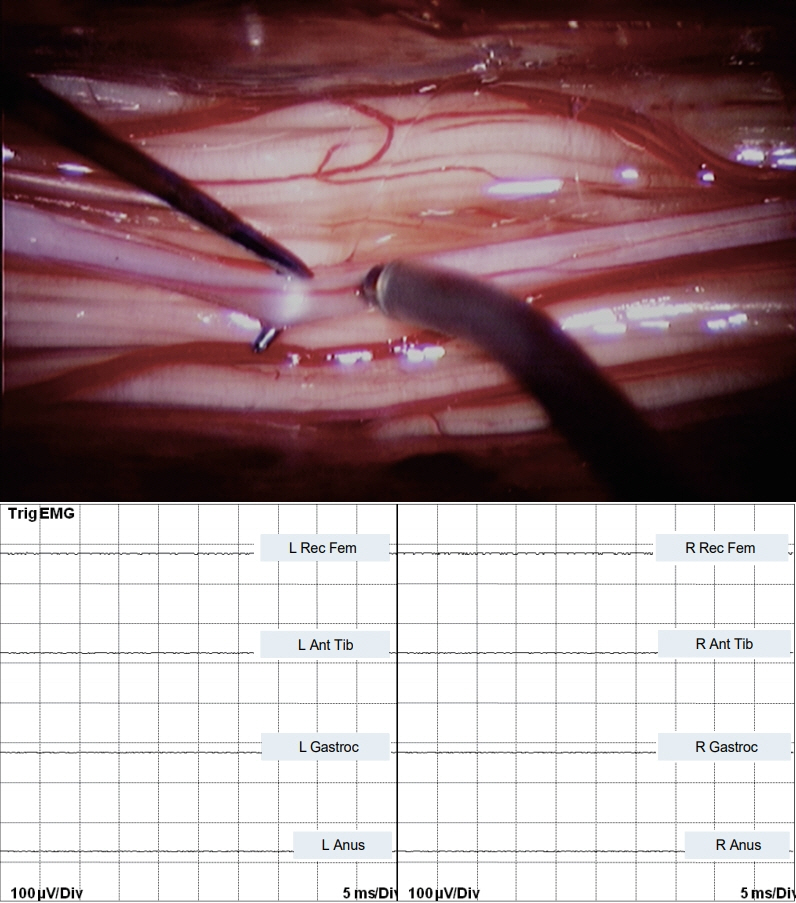
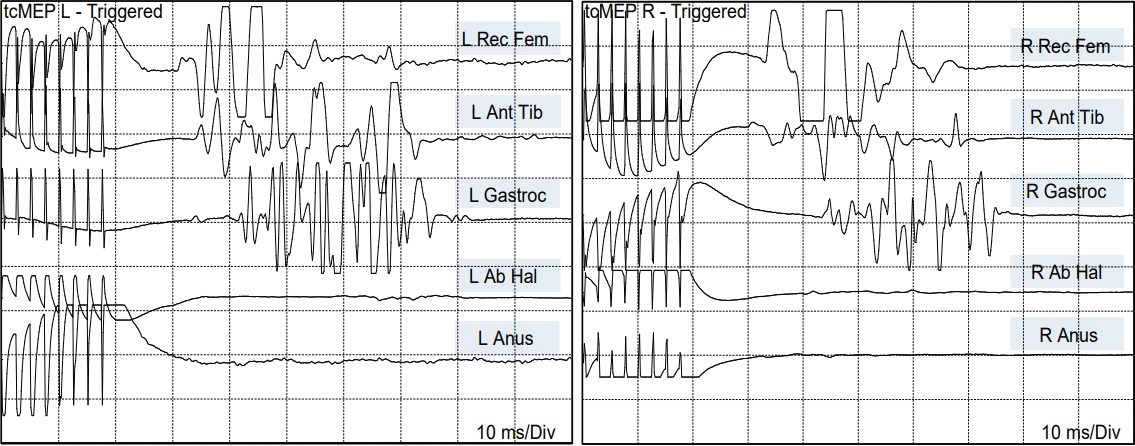
- Junctional neurulation represents the most recent adjunct to the well-known sequential embryological processes of primary and secondary neurulation. While its exact molecular processes, occurring at the end of primary and the beginning of secondary neurulation, are still being actively investigated, its pathological counterpart –junctional neural tube defect (JNTD)– had been described in 2017 based on three patients whose well-formed secondary neural tube, the conus, is widely separated from its corresponding primary neural tube and functionally disconnected from corticospinal control from above. Several other cases conforming to this bizarre neural tube arrangement have since appeared in the literature, reinforcing the validity of this entity. The cardinal clinical, neuroimaging, and electrophysiological features of JNTD, and the hypothesis of its embryogenetic mechanism, form part of this review.
Figure
Cited by 1 articles
-
Junctional Neural Tube Defect : Two Case Report
Bin Yuan, Shungen Huang, Xiangming Yan, Hangzhou Wang
J Korean Neurosurg Soc. 2025;68(1):105-109. doi: 10.3340/jkns.2024.0061.
Reference
-
References
1. Ali M, McNeely PD. Junctional neural tube defect: a supporting case report. Childs Nerv Syst. 34:1447–1448. 2018.
Article2. Beck CW, Slack JM. A developmental pathway controlling outgrowth of the Xenopus tail bud. Development. 126:1611–1620. 1999.
Article3. Catala M, Teillet MA, De Robertis EM, Le Douarin NM. A spinal cord fate map in the avian embryo: while regressing, Hensen’s node lays down the notochord and floor plate thus joining the spinal cord lateral walls. Development. 122:2599–2610. 1996.
Article4. Colas JF, Schoenwolf GC. Towards a cellular and molecular understanding of neurulation. Dev Dyn. 221:117–145. 2001.
Article5. Cooper O, Sweetman D, Wagstaff L, Munsterberg A. Expression of avian prickle genes during early development and organogenesis. Dev Dyn. 237:1442–1448. 2008.
Article6. Copp AJ, Greene ND. Genetics and development of neural tube defects. J Pathol. 220:217–230. 2010.
Article7. Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 4:784–793. 2003.
Article8. Criley BB. Analysis of the embryonic sources and mechanisms of development of posterior levels of chick neural tubes. J Morphol. 128:465–501. 1969.
Article9. Dady A, Blavet C, Duband JL. Timing and kinetics of E- to N-cadherin switch during neurulation in the avian embryo. Dev Dyn. 241:1333–1349. 2012.
Article10. Dady A, Havis E, Escriou V, Catala M, Duband JL. Junctional neurulation: a unique developmental program shaping a discrete region of the spinal cord highly susceptible to neural tube defects. J Neurosci. 34:13208–13221. 2014.
Article11. Doudney K, Ybot-Gonzalez P, Paternotte C, Stevenson RE, Greene ND, Moore GE, et al. Analysis of the planar cell polarity gene Vangl2 and its co-expressed paralogue Vangl1 in neural tube defect patients. Am J Med Genet A. 136:90–92. 2005.12. Dzamba BJ, Jakab KR, Marsden M, Schwartz MA, DeSimone DW. Cadherin adhesion, tissue tension, and noncanonical wnt signaling regulate fibronectin matrix organization. Dev Cell. 16:421–432. 2009.
Article13. Eibach S, Moes G, Hou YJ, Zovickian J, Pang D. Unjoined primary and secondary neural tubes: junctional neural tube defect, a new form of spinal dysraphism caused by disturbance of junctional neurulation. Childs Nerv Syst. 33:1633–1647. 2017.
Article14. Eibach S, Moes G, Zovickian J, Pang D. Limited dorsal myeloschisis associated with dermoid elements. Childs Nerv Syst. 33:55–67. 2017.
Article15. Florea SM, Faure A, Brunel H, Girard N, Scavarda D. A case of junctional neural tube defect associated with a lipoma of the filum terminale: a new subtype of junctional neural tube defect? J Neurosurg Pediatr. 21:601–605. 2018.
Article16. Goto T, Davidson L, Asashima M, Keller R. Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Curr Biol. 15:787–793. 2005.
Article17. Griffith CM, Wiley MJ, Sanders EJ. The vertebrate tail bud: three germ layers from one tissue. Anat Embryol (Berl). 185:101–113. 1992.
Article18. Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 88:49–92. 1951.
Article19. Hughes AF, Freeman RB. Comparative remarks on the development of the tail cord among higher vertebrates. J Embryol Exp Morphol. 32:355–363. 1974.
Article20. Kostović-Knezević L, Gajović S, Svajger A. Morphogenetic features in the tail region of the rat embryo. Int J Dev Biol. 35:191–195. 1991.21. Lowery LA, Sive H. Strategies of vertebrate neurulation and a re-evaluation of teleost neural tube formation. Mech Dev. 121:1189–1197. 2004.
Article22. Mills CL, Bellairs R. Mitosis and cell death in the tail of the chick embryo. Anat Embryol (Berl). 180:301–308. 1989.
Article23. Müller F, O’Rahilly R. The primitive streak, the caudal eminence and related structures in staged human embryos. Cells Tissues Organs. 177:2–20. 2004.
Article24. Nakaya Y, Kuroda S, Katagiri YT, Kaibuchi K, Takahashi Y. Mesenchymal-epithelial transition during somitic segmentation is regulated by differential roles of Cdc42 and Rac1. Dev Cell. 7:425–438. 2004.
Article25. Nievelstein RA, Hartwig NG, Vermeij-Keers C, Valk J. Embryonic development of the mammalian caudal neural tube. Teratology. 48:21–31. 1993.
Article26. O’Rahilly R, Müller F. Somites, spinal ganglia, and centra. Enumeration and interrelationships in staged human embryos, and implications for neural tube defects. Cells Tissues Organs. 173:75–92. 2003.27. Pang D. Sacral agenesis and caudal spinal cord malformations. Neurosurgery. 32:755–779. discussion 778-779. 1993.
Article28. Pang D, Zovickian J, Lee JY, Moes GS, Wang KC. Terminal myelocystocele: surgical observations and theory of embryogenesis. Neurosurgery. 70:1383–1404. discussion 1404-1405. 2012.29. Pang D, Zovickian J, Moes GS. Retained medullary cord in humans: late arrest of secondary neurulation. Neurosurgery. 68:1500–1519. discussion 1519. 2011.
Article30. Pang D, Zovickian J, Oviedo A. Long-term outcome of total and neartotal resection of spinal cord lipomas and radical reconstruction of the neural placode: part I-surgical technique. Neurosurgery. 65:511–528. discussion 528-529. 2009.31. Pang D, Zovickian J, Oviedo A, Moes GS. Limited dorsal myeloschisis: a distinctive clinicopathological entity. Neurosurgery. 67:1555–1579. discussion 1579-1580. 2010.
Article32. Pang D, Zovickian J, Wong ST, Hou YJ, Moes GS. Limited dorsal myeloschisis: a not-so-rare form of primary neurulation defect. Childs Nerv Syst. 29:1459–1484. 2013.
Article33. Saitsu H, Yamada S, Uwabe C, Ishibashi M, Shiota K. Development of the posterior neural tube in human embryos. Anat Embryol (Berl). 209:107–117. 2004.
Article34. Saraga-Babic M, Krolo M, Sapunar D, Terzic J, Biocic M. Differences in origin and fate between the cranial and caudal spinal cord during normal and disturbed human development. Acta Neuropathol. 91:194–199. 1996.
Article35. Schmidt C, Voin V, Iwanaga J, Alonso F, Oskouian RJ, Topale N, et al. Junctional neural tube defect in a newborn: report of a fourth case. Childs Nerv Syst. 33:873–875. 2017.
Article36. Schoenwolf GC. Histological and ultrastructural studies of secondary neurulation in mouse embryos. Am J Anat. 169:361–376. 1984.
Article37. Schoenwolf GC, Delongo J. Ultrastructure of secondary neurulation in the chick embryo. Am J Anat. 158:43–63. 1980.
Article38. Schoenwolf GC, Smith JL. Mechanisms of neurulation. Methods Mol Biol. 136:125–134. 2000.
Article39. Shimokita E, Takahashi Y. Secondary neurulation: fate-mapping and gene manipulation of the neural tube in tail bud. Dev Growth Differ. 53:401–410. 2011.
Article40. Tam PP. The histogenetic capacity of tissues in the caudal end of the embryonic axis of the mouse. J Embryol Exp Morphol. 82:253–266. 1984.
Article41. Tao H, Suzuki M, Kiyonari H, Abe T, Sasaoka T, Ueno N. Mouse prickle1, the homolog of a PCP gene, is essential for epiblast apical-basal polarity. Proc Natl Acad Sci U S A. 106:14426–14431. 2009.
Article42. Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 7:131–142. 2006.
Article43. Wang KC, Lee JS, Kim K, Im YJ, Park K, Kim KH, et al. Do junctional neural tube defect and segmental spinal dysgenesis have the same pathoembryological background? Childs Nerv Syst. 36:241–250. 2020.
Article44. Yang HJ, Wang KC, Chi JG, Lee MS, Lee YJ, Kim SK, et al. Cytokinetics of secondary neurulation in chick embryos: Hamburger and Hamilton stages 16-45. Childs Nerv Syst. 22:567–571. 2006.
Article45. Ybot-Gonzalez P, Savery D, Gerrelli D, Signore M, Mitchell CE, Faux CH, et al. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development. 134:789–799. 2007.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Junctional Neurulation : A Junction between Primary and Secondary Neural Tubes
- A Study of Homocysteine Metabolism related Neural Tube Defect
- The Characteristics of Fetus with Neural Tube Defect Accompanied by Club Foot in Prenatal Ultrasonography
- Re-closure Capacity of Surgically Induced Open Neural Tube Defect in Chick Embryos
- Recurrent Anencephaly in A Same Pregnant Women: Report of Two Cases