J Korean Neurosurg Soc.
2020 Jan;63(1):45-55. 10.3340/jkns.2019.0161.
Evaluation of Cumulative and Conditional Antibiotic Release from Vancomycin-Embedded Fibrin Sealant and Its Antibacterial Activity : An In Vitro Study
- Affiliations
-
- 1Department of Neurological Surgery, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea
- 2Neuroscience & Radiosurgery Hybrid Research Center, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea
- 3Department of Laboratory Medicine, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea
- 4Department of Neurosurgery, Inje University Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea
- KMID: 2501692
- DOI: http://doi.org/10.3340/jkns.2019.0161
Abstract
Objective
: Fibrin sealants have been used for hemostasis, sealant for cerebrospinal fluid leakage, and adhesive barrier in neurosurgery. Further, as its clinical use and role of an effective drug delivery vehicle have been proposed. This study was performed to measure antibacterial activity and continuous local antibiotic release from different concentrations of vancomycin-impregnated fibrin sealant in vitro.
Methods
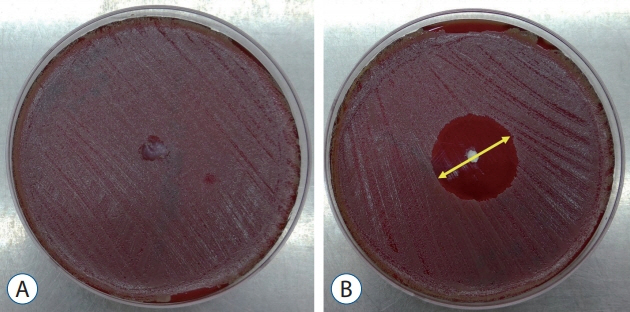
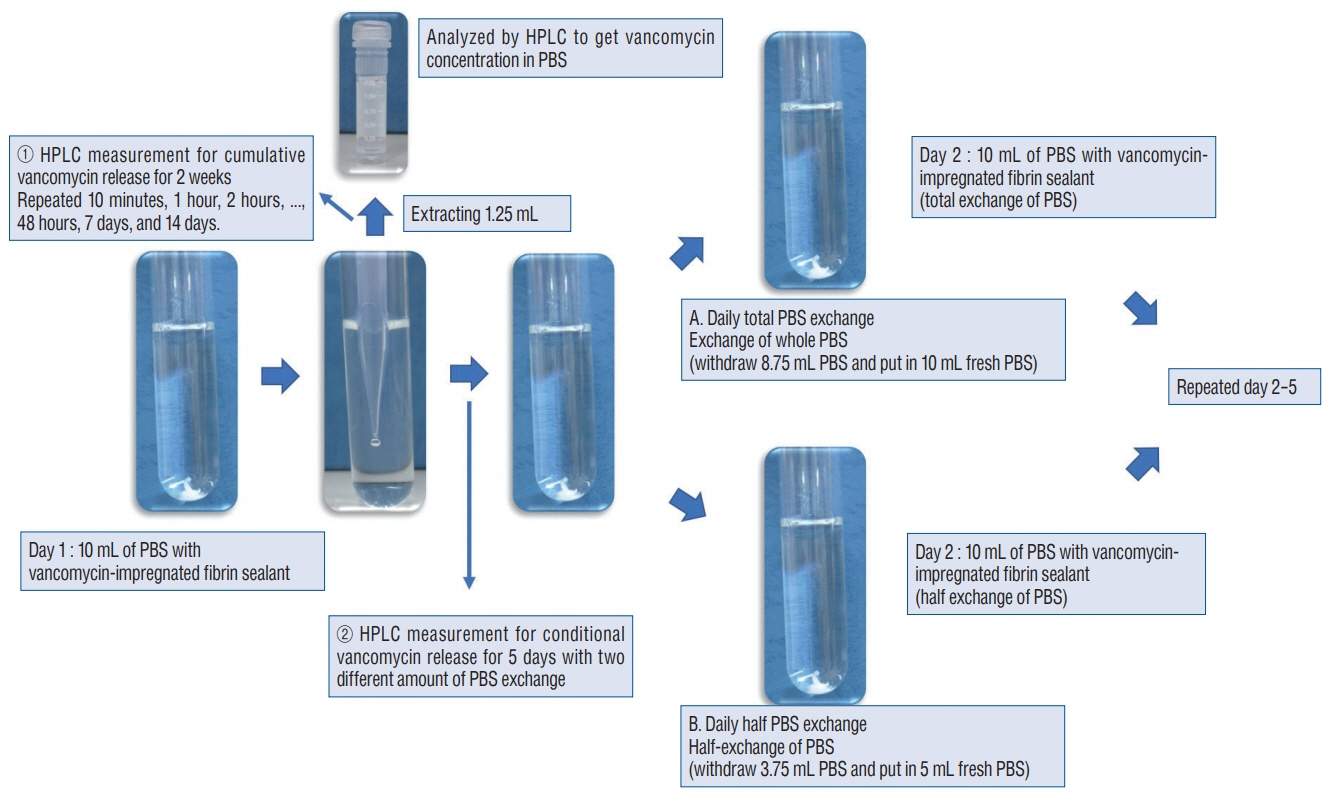
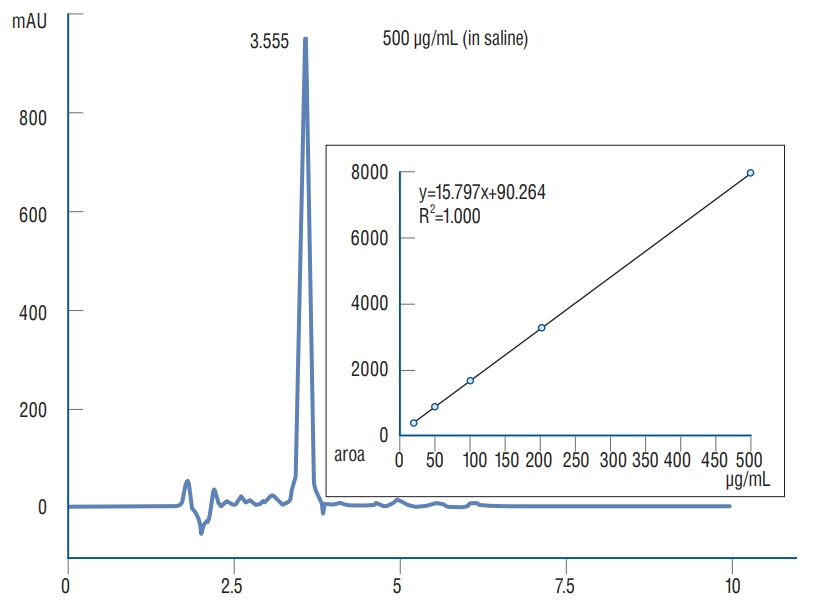
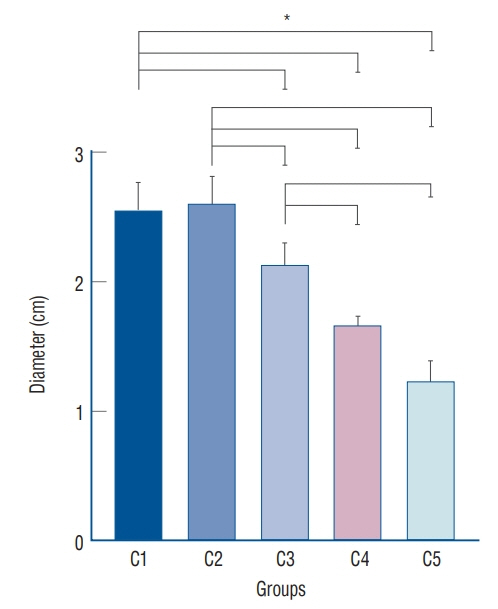
: Antibacterial activity was investigated by disk diffusion test by measuring the diameter of the growth inhibition zone of bacteria (methicillin-resistant Staphylococcus aureus, ATCC29213) from vancomycin-embedded fibrin sealant disc diluted at five different concentrations (C1–C5; 8.33, 4.167, 0.83, 0.083, and 0.0083 mg/disc, respectively). Continuous and conditioned release of vancomycin concentration (for 2 weeks and for 5 days, respectively) were also measured using high-performance liquid chromatography (HPLC) method. To mimic the physiologic wound conditions with in vitro, conditioned vancomycin release in phosphate buffer solution (PBS) was measured and replaced PBS for five consecutive days, half a day or completely daily.
Results
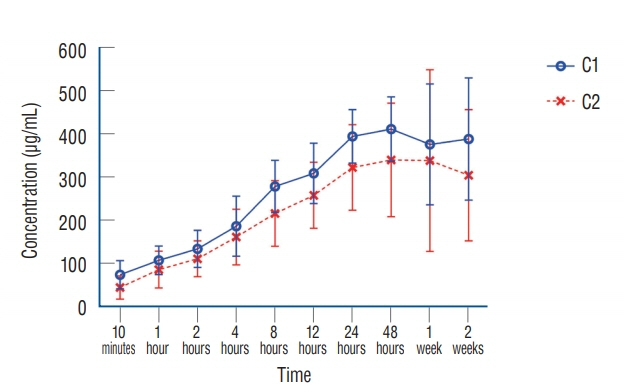
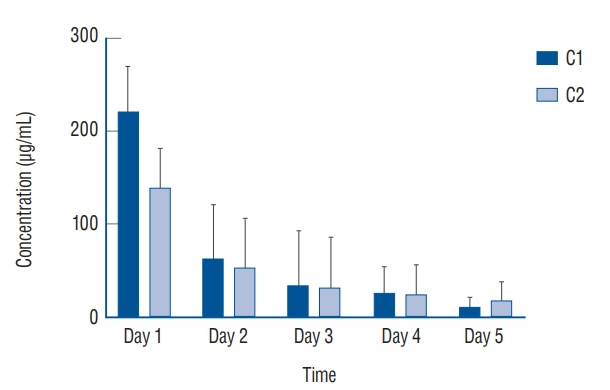
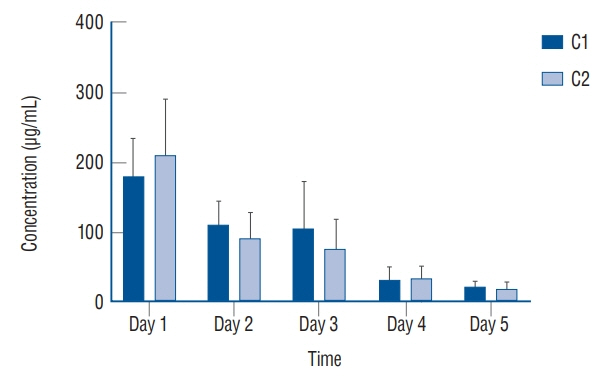
: In the disk diffusion test, the mean diameters of bacterial inhibition zone were 2.54±0.07 cm, 2.61±0.12 cm, and 2.13±0.15 cm (C1, C2, and C3 respectively) but 1.67±0.06 cm and 1.23±0.15 cm in C4 and C5, respectively. Continuous elution test elicited the peak release of vancomycin from the fibrin sealant at 48 hours, with continued release until 2 weeks. However, conditioned vancomycin release decreased to half or more on day 2, however, the sustainable release was measured over the therapeutic dose (10–20 μg/mL) for 5 days and 4 days in assays of half and total exchange of PBS.
Conclusion
: This study suggests that fibrin sealant can provide an efficient vehicle for antibiotic drug release in a wide range of neurosurgical procedures and the safe and effective therapeutic dose will be at the concentration embedded of 4.167 mg/disc or more of vancomycin.
Keyword
Figure
Reference
-
References
1. Abdullah KG, Attiah MA, Olsen AS, Richardson A, Lucas TH. Reducing surgical site infections following craniotomy: examination of the use of topical vancomycin. J Neurosurg. 123:1600–1604. 2015.
Article2. Ahmad E, Fatima MT, Hoque M, Owais M, Saleemuddin M. Fibrin matrices: the versatile therapeutic delivery systems. Int J Biol Macromol. 81:121–136. 2015.
Article3. Anderson PA, Savage JW, Vaccaro AR, Radcliff K, Arnold PM, Lawrence BD, et al. Prevention of surgical site infection in spine surgery. Neurosurgery. 80(3S):S114–S123. 2017.
Article4. Aziz KT, Best MJ, Naseer Z, Skolasky RL, Ponnusamy KE, Sterling RS, et al. the association of delirium with perioperative complications in primary elective total hip arthroplasty. Clin Orthop Surg. 10:286–291. 2018.
Article5. Bernatz JT, Anderson PA. Thirty-day readmission rates in spine surgery: systematic review and meta-analysis. Neurosurg Focus. 39:E7. 2015.
Article6. Bhattacharya M, Wozniak DJ, Stoodley P, Hall-Stoodley L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev Anti Infect Ther. 13:1499–1516. 2015.7. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 14:244–269. 2001.
Article8. Cashman JD, Jackson JK, Mugabe C, Gilchrist S, Ball K, Tredwell S, et al. The use of tissue sealants to deliver antibiotics to an orthopaedic surgical site with a titanium implant. J Orthop Sci. 18:165–174. 2013.
Article9. Charlton-Ouw KM, Kubrusly F, Sandhu HK, Swick MC, Leake SS, Gulbis BE, et al. In vitro efficacy of antibiotic beads in treating abdominal vascular graft infections. J Vasc Surg. 62:1048–1053. 2015.
Article10. Dessy AM, Yuk FJ, Maniya AY, Connolly JG, Nathanson JT, Rasouli JJ, et al. Reduced surgical site infection rates following spine surgery using an enhanced prophylaxis protocol. Cureus. 9:e1139. 2017.11. Emohare O, Ledonio CG, Hill BW, Davis RA, Polly DW Jr, Kang MM. Cost savings analysis of intrawound vancomycin powder in posterior spinal surgery. Spine J. 14:2710–2715. 2014.
Article12. Eren B, Karagöz Güzey F, Kitis S, Özkan N, Korkut C. The effectiveness of pedicle screw immersion in vancomycin and ceftriaxone solution for the prevention of postoperative spinal infection: a prospective comparative study. Acta Orthop Traumatol Turc. 52:289–293. 2018.
Article13. Grabel ZJ, Boden A, Segal DN, Boden S, Milby AH, Heller JG. The impact of prophylactic intraoperative vancomycin powder on microbial profile, antibiotic regimen, length of stay, and reoperation rate in elective spine surgery. Spine J. 19:261–266. 2018.
Article14. Hake ME, Young H, Hak DJ, Stahel PF, Hammerberg EM, Mauffrey C. Local antibiotic therapy strategies in orthopaedic trauma: practical tips and tricks and review of the literature. Injury. 46:1447–1456. 2015.
Article15. Hill BW, Emohare O, Song B, Davis R, Kang MM. The use of vancomycin powder reduces surgical reoperation in posterior instrumented and noninstrumented spinal surgery. Acta Neurochir (Wien). 156:749–754. 2014.
Article16. Horowitz B, Busch M. Estimating the pathogen safety of manufactured human plasma products: application to fibrin sealants and to thrombin. Transfusion. 48:1739–1753. 2008.
Article17. Jennings JA, Carpenter DP, Troxel KS, Beenken KE, Smeltzer MS, Courtney HS, et al. Novel antibiotic-loaded point-of-care implant coating inhibits biofilm. Clin Orthop Relat Res. 473:2270–2282. 2015.
Article18. Khan NR, Thompson CJ, DeCuypere M, Angotti JM, Kalobwe E, Muhlbauer MS, et al. A meta-analysis of spinal surgical site infection and vancomycin powder. J Neurosurg Spine. 21:974–983. 2014.
Article19. Kroez M, Lang W, Dickneite G. Wound healing and degradation of the fibrin sealant Beriplast P following partial liver resection in rabbits. Wound Repair Regen. 13:318–323. 2005.
Article20. Kummer A, Tafin UF, Borens O. Effect of sonication on the elution of antibiotics from polymethyl methacrylate (PMMA). J Bone Jt Infect. 2:208–212. 2017.
Article21. McConoughey SJ, Howlin RP, Wiseman J, Stoodley P, Calhoun JH. Comparing PMMA and calcium sulfate as carriers for the local delivery of antibiotics to infected surgical sites. J Biomed Mater Res B Appl Biomater. 103:870–877. 2015.
Article22. Mintz PD, Mayers L, Avery N, Flanagan HL, Burks SG, Spotnitz WD. Fibrin sealant: clinical use and the development of the University of Virginia Tissue Adhesive Center. Ann Clin Lab Sci. 31:108–118. 2001.23. Morishima M, Marui A, Yanagi S, Nomura T, Nakajima N, Hyon SH, et al. Sustained release of vancomycin from a new biodegradable glue to prevent methicillin-resistant Staphylococcus aureus graft infection. Interact Cardiovasc Thorac Surg. 11:52–55. 2010.
Article24. Najjar PA, Smink DS. Prophylactic antibiotics and prevention of surgical site infections. Surg Clin North Am. 95:269–283. 2015.
Article25. Ozaki S, Saito A, Nakaminami H, Ono M, Noguchi N, Motomura N. Comprehensive evaluation of fibrin glue as a local drug-delivery systemefficacy and safety of sustained release of vancomycin by fibrin glue against local methicillin-resistant Staphylococcus aureus infection. J Artif Organs. 17:42–49. 2014.
Article26. Percival SL. Importance of biofilm formation in surgical infection. Br J Surg. 104:e85–e94. 2017.
Article27. Sekhar LN, Natarajan SK, Manning T, Bhagawati D. The use of fibrin glue to stop venous bleeding in the epidural space, vertebral venous plexus, and anterior cavernous sinus: technical note. Neurosurgery. 61(3 Suppl):E51. discussion E51. 2007.28. Spotnitz WD. Fibrin sealant: past, present, and future: a brief review. World J Surg. 34:632–634. 2010.
Article29. Strom RG, Pacione D, Kalhorn SP, Frempong-Boadu AK. Decreased risk of wound infection after posterior cervical fusion with routine local application of vancomycin powder. Spine (Phila Pa 1976). 38:991–994. 2013.
Article30. Theologis AA, Demirkiran G, Callahan M, Pekmezci M, Ames C, Deviren V. Local intrawound vancomycin powder decreases the risk of surgical site infections in complex adult deformity reconstruction: a cost analysis. Spine (Phila Pa 1976). 39:1875–1880. 2014.
Article31. Tofuku K, Koga H, Yanase M, Komiya S. The use of antibiotic-impregnated fibrin sealant for the prevention of surgical site infection associated with spinal instrumentation. Eur Spine J. 21:2027–2033. 2012.
Article32. Toyooka T, Otani N, Wada K, Tomiyama A, Ueno H, Fujii K, et al. Effect of fibrin glue injection into the cavernous sinus for hemostasis during transcavernous surgery on the cerebral venous draining system. Oper Neurosurg (Hagerstown). 13:224–231. 2017.
Article33. Yang L, Wu J, Zhang G, Zhao Z, Fan Z, Wang B. A novel technology: percutaneous injection of fibrin glue as a treatment for frontal sinus cerebrospinal fluid rhinorrhea. J Craniofac Surg. 24:1646–1649. 2013.34. Yeramaneni S, Robinson C, Hostin R. Impact of spine surgery complications on costs associated with management of adult spinal deformity. Curr Rev Musculoskelet Med. 9:327–332. 2016.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Vitro Pharmacokinetics of Vancomycin Release from Locally Implantable Materials
- Does Fibrin Sealant Reduce Seroma after Immediate Breast Reconstruction Utilizing a Latissimus Dorsi Myocutaneous Flap?
- Experimental Study on the Effect of Fibrin Sealant in Renal Surgery
- Fibrin Sealant Injection for Control of Hemorrhage in Vitreous Surgery of Proliferative Diabetic Retinopathy
- Antibacterial Efficacy of Dental Sealant Containing Phytoncide