J Pathol Transl Med.
2020 Jan;54(1):95-102. 10.4132/jptm.2019.10.24.
Clinicopathologic characteristics of HER2-positive pure mucinous carcinoma of the breast
- Affiliations
-
- 1Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University College of Medicine, Seoul, Korea
- 22Division of Breast and Endocrine Surgery, Specialized Surgical Unit, King Abdullah Medical City, Makkah, Saudi Arabia
- 3Division of Breast Surgery, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2501597
- DOI: http://doi.org/10.4132/jptm.2019.10.24
Abstract
- Background
Pure mucinous carcinoma (PMC) is a rare type of breast cancer, estimated to represent 2% of invasive breast cancer. PMC is typically positive for estrogen receptors (ER) and progesterone receptors (PR) and negative for human epidermal growth factor receptor 2 (HER2). The clinicopathologic characteristics of HER2-positive PMC have not been investigated.
Methods
Pathology archives were searched for PMC diagnosed from January 1999 to April 2018. Clinicopathologic data and microscopic findings were reviewed and compared between HER2-positive PMC and HER2-negative PMC. We also analyzed the differences in disease-free survival (DFS) and overall survival according to clinicopathologic parameters including HER2 status in overall PMC cases.
Results
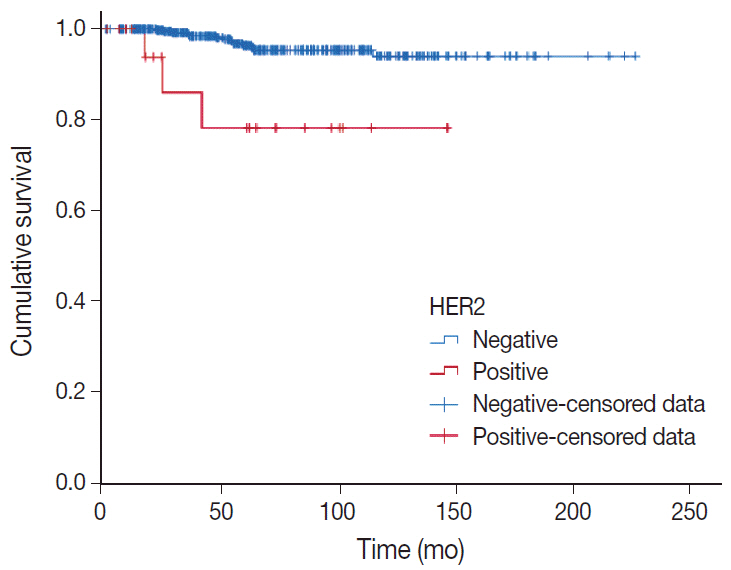
There were 21 HER2-positive cases (4.8%) in 438 PMCs. The average tumor size of HER2-positive PMC was 32.21 mm (± 26.55). Lymph node metastasis was present in seven cases. Compared to HER2-negative PMC, HER2-positive PMC presented with a more advanced T category (p < .001), more frequent lymph node metastasis (p = .009), and a higher nuclear and histologic grade (p < .001). Microscopically, signet ring cells were frequently observed in HER2-positive PMC (p < .001), whereas a micropapillary pattern was more frequent in HER2-negative PMC (p = .012). HER2-positive PMC was more frequently negative for ER (33.3% vs. 1.2%) and PR (28.6% vs. 7.2%) than HER2-negative PMC and showed a high Ki-67 labeling index. During follow-up, distant metastasis and recurrence developed in three HER2-positive PMC patients. Multivariate analysis revealed that only HER2-positivity and lymph node status were significantly associated with DFS.
Conclusions
Our results suggest that HER2-positive PMC is a more aggressive subgroup of PMC. HER2 positivity should be considered for adequate management of PMC.
Figure
Reference
-
1. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO classification of tumours of the breast. 4th ed. Lyon: IARC Press;2012.2. Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008; 111:541–7.
Article3. Barkley CR, Ligibel JA, Wong JS, Lipsitz S, Smith BL, Golshan M. Mucinous breast carcinoma: a large contemporary series. Am J Surg. 2008; 196:549–51.
Article4. Lacroix-Triki M, Suarez PH, MacKay A, et al. Mucinous carcinoma of the breast is genomically distinct from invasive ductal carcinomas of no special type. J Pathol. 2010; 222:282–98.
Article5. Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017; 389:2415–29.
Article6. Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ, Yang JH. Mucinous carcinoma of the breast in comparison with invasive ductal carcinoma: clinicopathologic characteristics and prognosis. J Breast Cancer. 2011; 14:308–13.
Article7. Pan B, Yao R, Shi J, et al. Prognosis of subtypes of the mucinous breast carcinoma in Chinese women: a population-based study of 32-year experience (1983-2014). Oncotarget. 2016; 7:38864–75.
Article8. Ranade A, Batra R, Sandhu G, Chitale RA, Balderacchi J. Clinicopathological evaluation of 100 cases of mucinous carcinoma of breast with emphasis on axillary staging and special reference to a micropapillary pattern. J Clin Pathol. 2010; 63:1043–7.
Article9. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010; 28:2784–95.
Article10. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31:3997–4013.11. Fu J, Wu L, Jiang M, et al. Clinical nomogram for predicting survival outcomes in early mucinous breast cancer. PLoS One. 2016; 11:e0164921.
Article12. Avisar E, Khan MA, Axelrod D, Oza K. Pure mucinous carcinoma of the breast: a clinicopathologic correlation study. Ann Surg Oncol. 1998; 5:447–51.
Article13. Burstein HJ. The distinctive nature of HER2-positive breast cancers. N Engl J Med. 2005; 353:1652–4.
Article14. Gwark SC, Lee HS, Lee Y, et al. Clinical implication of HER2 status in hormone receptor-positive mucinous breast cancer. Ann Surg Oncol. 2019; 26:2166–74.
Article15. Jang Y, Cho EY, Cho SY. Human epidermal growth factor receptor 2-positive mucinous carcinoma with signet ring cell differentiation, which showed complete response after neoadjuvant chemotherapy. J Breast Cancer. 2019; 22:336–40.
Article16. Kim HM, Kim EK, Koo JS. Mucinous carcinoma with extensive signet ring cell differentiation: a case report. J Pathol Transl Med. 2017; 51:176–9.
Article17. Leung KM, Yeoh GP, Chan JK, Cheung PS, Chan KW. Ductal type signet ring cell carcinoma of breast with growth pattern of pure mucinous carcinoma. Pathology. 2011; 43:282–4.
Article18. Varga Z, Zhao J, Ohlschlegel C, Odermatt B, Heitz PU. Preferential HER-2/neu overexpression and/or amplification in aggressive histological subtypes of invasive breast cancer. Histopathology. 2004; 44:332–8.
Article19. Barbashina V, Corben AD, Akram M, Vallejo C, Tan LK. Mucinous micropapillary carcinoma of the breast: an aggressive counterpart to conventional pure mucinous tumors. Hum Pathol. 2013; 44:1577–85.
Article20. Kim HJ, Park K, Kim JY, Kang G, Gwak G, Park I. Prognostic significance of a micropapillary pattern in pure mucinous carcinoma of the breast: comparative analysis with micropapillary carcinoma. J Pathol Transl Med. 2017; 51:403–9.
Article21. Xu X, Bi R, Shui R, et al. Micropapillary pattern in pure mucinous carcinoma of the breast: does it matter or not? Histopathology. 2019; 74:248–55.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and Treatment of HER2-Positive Breast Cancer
- Human Epidermal Growth Factor Receptor 2-positive Mucinous Carcinoma with Signet Ring Cell Differentiation, Which Showed Complete Response after Neoadjuvant Chemotherapy
- Cancer Subtypes of Breast Carcinoma with Micropapillary and Mucinous Component Based on Immunohistochemical Profile
- Prognostic Significance of a Micropapillary Pattern in Pure Mucinous Carcinoma of the Breast: Comparative Analysis with Micropapillary Carcinoma
- The Expression of Glut-1, CAIX, and MCT4 in Mucinous Carcinoma