Yeungnam Univ J Med.
2020 Apr;37(2):73-78. 10.12701/yujm.2019.00437.
Function of hepatocyte growth factor in gastric cancer proliferation and invasion
- Affiliations
-
- 1Department of Hematology-Oncology, Yeungnam University College of Medicine, Daegu, Korea
- KMID: 2501412
- DOI: http://doi.org/10.12701/yujm.2019.00437
Abstract
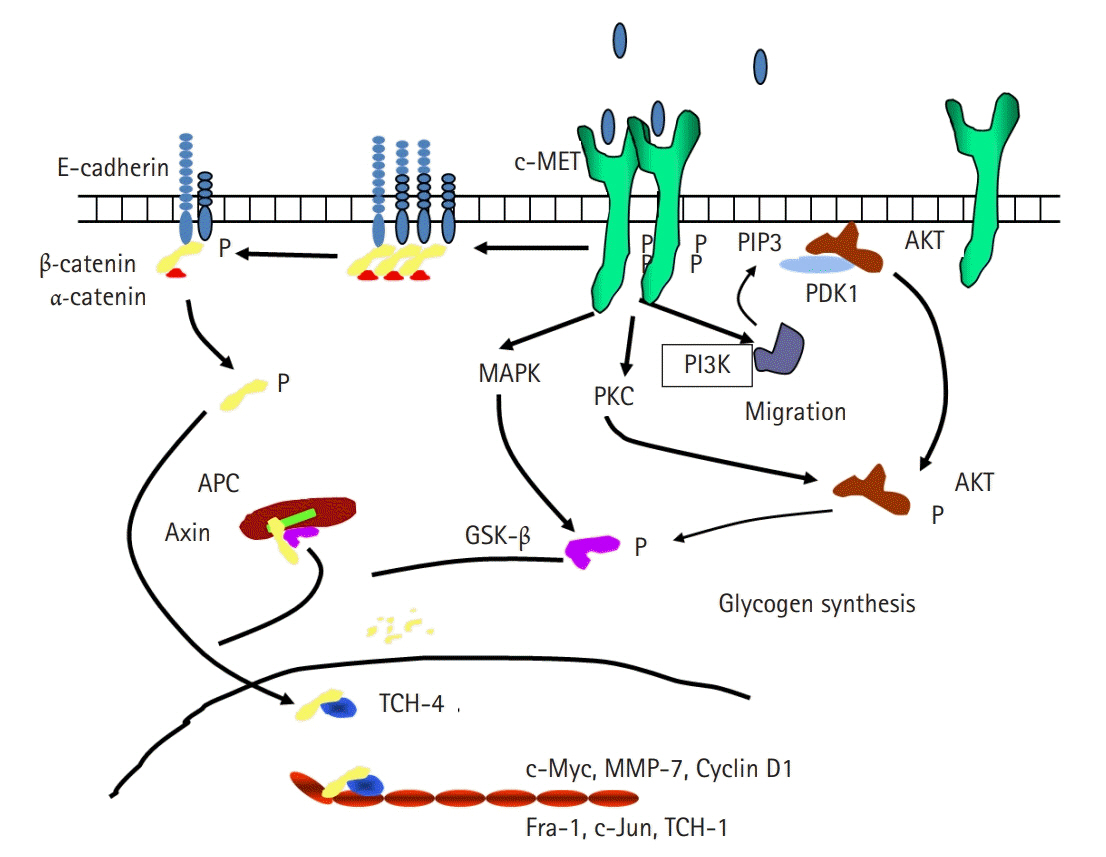
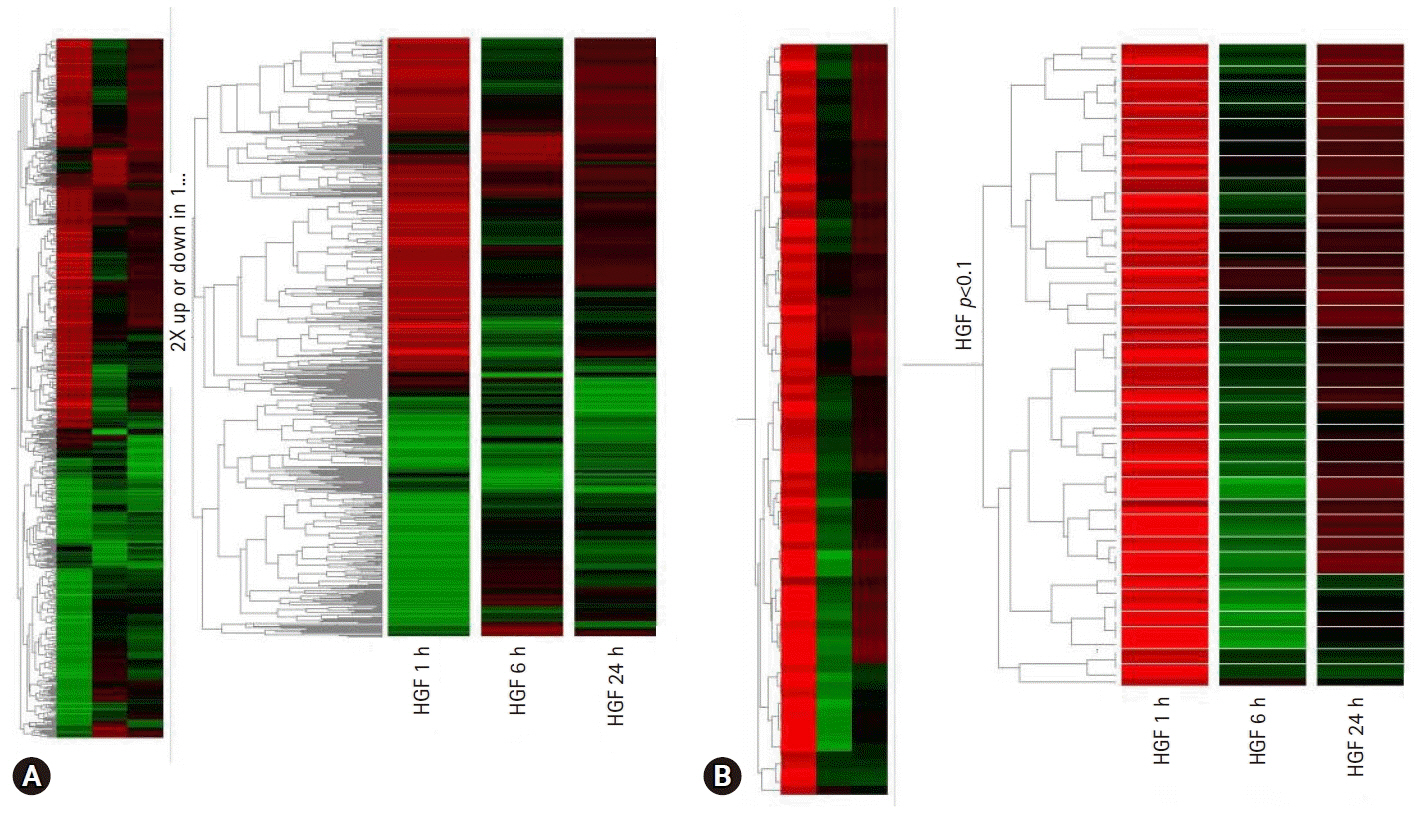
- Cancer incidence has been increasing steadily and is the leading cause of mortality worldwide. Gastric cancer is still most common malignancy in Korea. Cancer initiation and progression are multistep processes involving various growth factors and their ligands. Among these growth factors, we have studied hepatocyte growth factor (HGF), which is associated with cell proliferation and invasion, leading to cancer and metastasis, especially in gastric cancer. We explored the intercellular communication between HGF and other surface membrane receptors in gastric cancer cell lines. Using complimentary deoxyribonucleic acid microarray technology, we found new genes associated with HGF in the stomach cancer cell lines, NUGC-3 and MKN-28, and identified their function within the HGF pathway. The HGF/N-methyl-N’-nitroso-guanidine human osteosarcoma transforming gene (c-MET) axis interacts with several molecules including E-cadherin, urokinase plasminogen activator, KiSS-1, Jun B, and lipocalin-2. This pathway may affect cell invasion and metastasis or cell apoptosis and is therefore associated with tumorigenesis and metastasis in gastric cancer.
Figure
Reference
-
References
1. Veikkola T, Alitalo K. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 1999; 9:211–20.
Article2. Schmitt FC, Soares R. TGF-alpha and angiogenesis. Am J Surg Pathol. 1999; 23:358–9.3. Botta M, Manetti F, Corelli F. Fibroblast growth factors and their inhibitors. Curr Pharm Des. 2000; 6:1897–924.
Article4. Mooradian DL, Diglio CA. Production of a transforming growth factor-beta-like growth factor by RSV-transformed rat cerebral microvascular endothelial cells. Tumour Biol. 1991; 12:171–83.
Article5. Lamszus K, Jin L, Fuchs A, Shi E, Chowdhury S, Yao Y, et al. Scatter factor stimulates tumor growth and tumor angiogenesis in human breast cancers in the mammary fat pads of nude mice. Lab Invest. 1997; 76:339–53.6. Desbaillets I, Diserens AC, Tribolet N, Hamou MF, Van Meir EG. Upregulation of interleukin 8 by oxygen-deprived cells in glioblastoma suggests a role in leukocyte activation, chemotaxis, and angiogenesis. J Exp Med. 1997; 186:1201–12.
Article7. Woodburn JR. The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol Ther. 1999; 82:241–50.
Article8. Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995; 373:699–702.
Article9. Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010; 11:834–48.
Article10. Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012; 12:89–103.
Article11. Maulik G, Shrikhande A, Kijima T, Ma PC, Morrison PT, Salgia R. Role of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibition. Cytokine Growth Factor Rev. 2002; 13:41–59.
Article12. Shin SJ, Kim KO, Kim MK, Lee KH, Hyun MS, Kim KJ, et al. Expression of E-cadherin and uPA and their association with the prognosis of pancreatic cancer. Jpn J Clin Oncol. 2005; 35:342–8.
Article13. Chen WC, Obrink B. Cell-cell contacts mediated by E-cadherin (uvomorulin) restrict invasive behavior of L-cells. J Cell Biol. 1991; 114:319–27.
Article14. Althaus E, Karotke E, Nitsch K, Winkler H. An experimental re-examination of the upper stability limit of muscovite plus quartz. Neues Jahrb Miner Monatsh. 1970; 7:325–36.15. Hiscox S, Jiang WG. Hepatocyte growth factor/scatter factor disrupts epithelial tumour cell-cell adhesion: involvement of beta-catenin. Anticancer Res. 1999; 19:509–17.16. Lee KH, Choi EY, Hyun MS, Jang BI, Kim TN, Kim SW, et al. Association of extracellular cleavage of E-cadherin mediated by MMP-7 with HGF-induced in vitro invasion in human stomach cancer cells. Eur Surg Res. 2007; 39:208–15.
Article17. Markus G, Takita H, Camiolo SM, Corasanti JG, Evers JL, Hobika GH. Content and characterization of plasminogen activators in human lung tumors and normal lung tissue. Cancer Res. 1980; 40:841–8.18. Sappino AP, Busso N, Belin D, Vassalli JD. Increase of urokinase-type plasminogen activator gene expression in human lung and breast carcinomas. Cancer Res. 1987; 47:4043–6.19. Markus G, Camiolo SM, Kohga S, Madeja JM, Mittelman A. Plasminogen activator secretion of human tumors in short-term organ culture, including a comparison of primary and metastatic colon tumors. Cancer Res. 1983; 43:5517–25.20. Alonso DF, Farias EF, Ladeda V, Davel L, Puricelli L, Bal de Kier Joffe E. Effects of synthetic urokinase inhibitors on local invasion and metastasis in a murine mammary tumor model. Breast Cancer Res Treat. 1996; 40:209–23.
Article21. Kruger A, Soeltl R, Lutz V, Wilhelm OG, Magdolen V, Rojo EE, et al. Reduction of breast carcinoma tumor growth and lung colonization by overexpression of the soluble urokinase-type plasminogen activator receptor (CD87). Cancer Gene Ther. 2000; 7:292–9.
Article22. Lee KH, Bae SH, Lee JL, Hyun MS, Kim SH, Song SK, et al. Relationship between urokinase-type plasminogen receptor, interleukin-8 gene expression and clinicopathological features in gastric cancer. Oncology. 2004; 66:210–7.
Article23. Lee KH, Kim SW, Kim JR. Reactive oxygen species regulate urokinase plasminogen activator expression and cell invasion via mitogen-activated protein kinase pathways after treatment with hepatocyte growth factor in stomach cancer cells. J Exp Clin Cancer Res. 2009; 28:73.
Article24. Lee KH, Choi EY, Kim MK, Kim KO, Jang BI, Kim SW, et al. Inhibition of histone deacetylase activity down-regulates urokinase plasminogen activator and matrix metalloproteinase-9 expression in gastric cancer. Mol Cell Biochem. 2010; 343:163–71.
Article25. Lee KH, Choi EY, Koh SA, Kim MK, Kim KO, Lee SH, et al. Down-regulation of survivin suppresses uro-plasminogen activator through transcription factor JunB. Exp Mol Med. 2011; 43:501–9.
Article26. Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995; 80:285–91.
Article27. Lee KH, Choi EY, Kim MK, Hyun MS, Eun JR, Jang BI, et al. Hepatocyte growth factor promotes cell survival by phosphorylation of BAD in gastric cancer cells. Oncol Res. 2008; 17:23–32.
Article28. Lee JH, Welch DR. Identification of highly expressed genes in metastasis-suppressed chromosome 6/human malignant melanoma hybrid cells using subtractive hybridization and differential display. Int J Cancer. 1997; 71:1035–44.
Article29. Lee JH, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 1997; 57:2384–7.30. Martin TA, Watkins G, Jiang WG. KiSS-1 expression in human breast cancer. Clin Exp Metastasis. 2005; 22:503–11.
Article31. Lee KH, Kim JR. Kiss-1 suppresses MMP-9 expression by activating p38 MAP kinase in human stomach cancer. Oncol Res. 2009; 18:107–16.
Article32. Ozanne BW, Spence HJ, McGarry LC, Hennigan RF. Transcription factors control invasion: AP-1 the first among equals. Oncogene. 2007; 26:1–10.
Article33. Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003; 3:859–68.
Article34. Vandel L, Pfarr CM, Huguier S, Loiseau L, Sergeant A, Castellazzi M. Increased transforming activity of JunB and JunD by introduction of an heterologous homodimerization domain. Oncogene. 1995; 10:495–507.35. Tschesche H, Zolzer V, Triebel S, Bartsch S. The human neutrophil lipocalin supports the allosteric activation of matrix metalloproteinases. Eur J Biochem. 2001; 268:1918–28.
Article36. Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001; 276:37258–65.37. Lee KH, Kim JR. Regulation of HGF-mediated cell proliferation and invasion through NF-κB, JunB, and MMP-9 cascades in stomach cancer cells. Clin Exp Metastasis. 2012; 29:263–72.
Article38. Bratt T, Ohlson S, Borregaard N. Interactions between neutrophil gelatinase-associated lipocalin and natural lipophilic ligands. Biochim Biophys Acta. 1999; 1472:262–9.
Article39. Koh SA, Lee KH. HGF mediated upregulation of lipocalin 2 regulates MMP9 through nuclear factor-κB activation. Oncol Rep. 2015; 34:2179–87.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Serum Hepatocyte Growth Factor in Gastric Cancer Patients
- Effect of Hepatocyte Growth Factor on the Expression of E-cadherin in Gastric Carcinoma Cell Lines
- Expression of Met Protein in Colorectal Carcinoma
- Changes of the Serum Hepatocyte Growth Factor Levels after Hepatectomy
- Significant Correlation of Hepatocyte Growth Factor Level with Progression of Gastric Adenocarcinoma