Cancer Res Treat.
2020 Jan;52(1):309-319. 10.4143/crt.2019.161.
Characterization of Oncolytic Vaccinia Virus Harboring the Human IFNB1 and CES2 Transgenes
- Affiliations
-
- 1Department of Pharmacology and Medical Research Center (MRC), Pusan National University School of Medicine, Yangsan, Korea
- 2Department of Pharmacy and Pusan Cancer Research Center, Pusan National University, Busan, Korea
- 3Department of Microbiology and Immunology, Pusan National University School of Medicine, Yangsan, Korea
- 4School of Pharmaceutical Sciences (Shenzhen), Sun Yat-sen University, Guangzhou, China
- KMID: 2501223
- DOI: http://doi.org/10.4143/crt.2019.161
Abstract
- Purpose
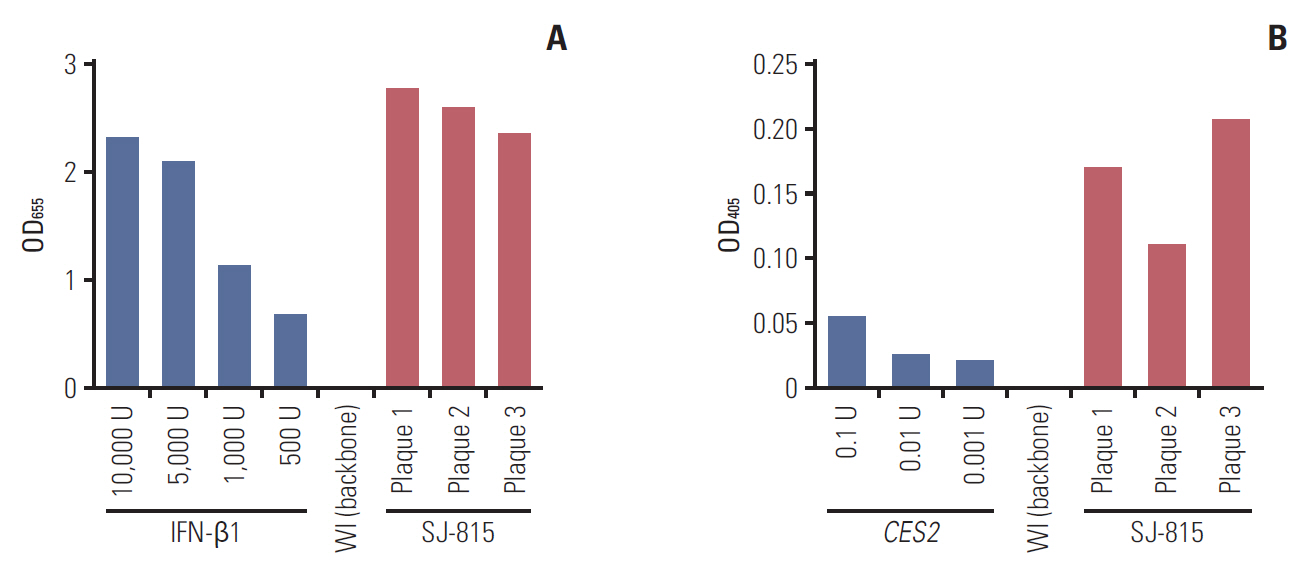
The purpose of this study was to assess characteristics of SJ-815, a novel oncolytic vaccinia virus lacking a functional thymidine kinase-encoding TK gene, and instead, having two human transgenes: the IFNB1 that encodes interferon β1, and the CES2 that encodes carboxylesterase 2, which metabolizes the prodrug, irinotecan, into cytotoxic SN-38.
Materials and Methods
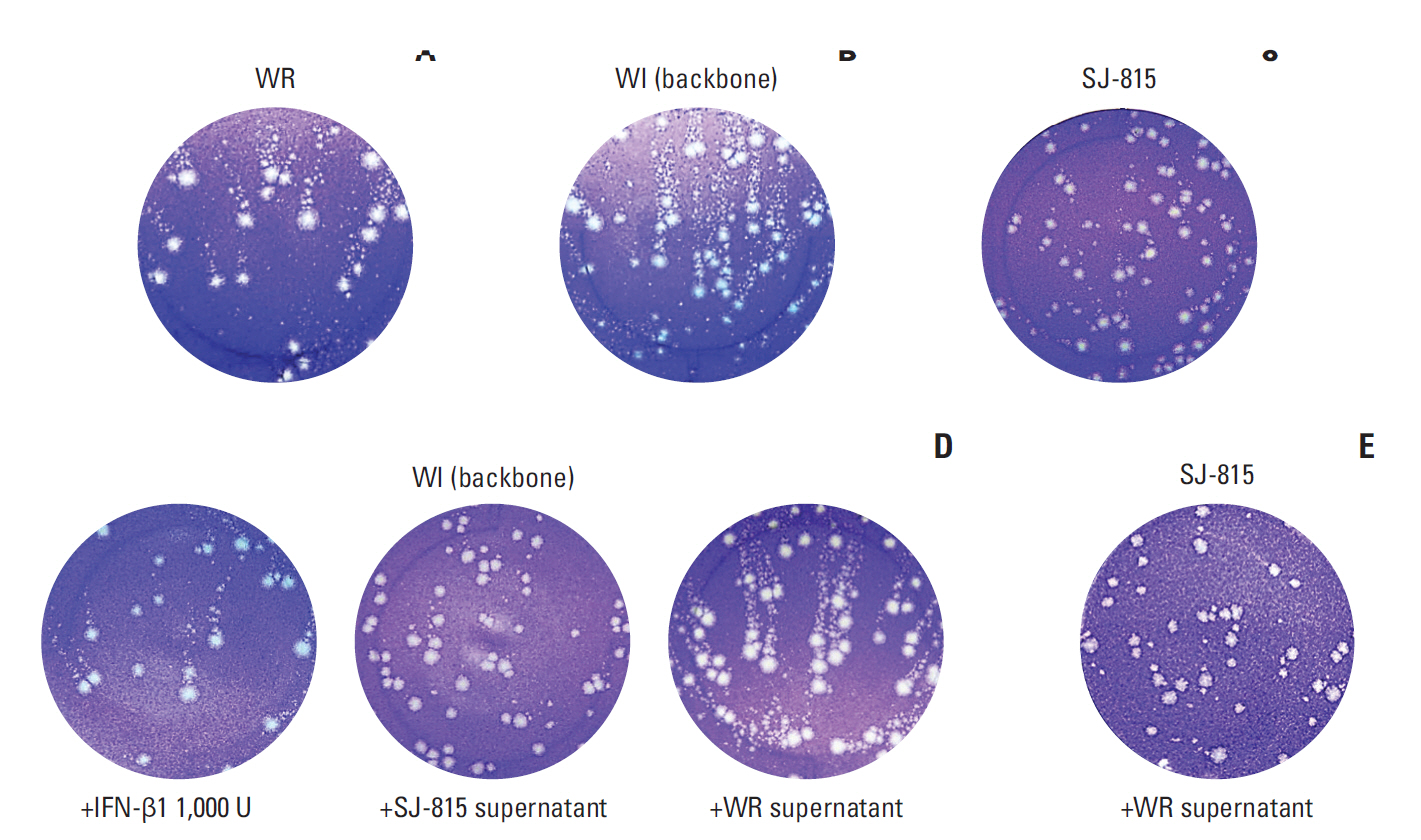
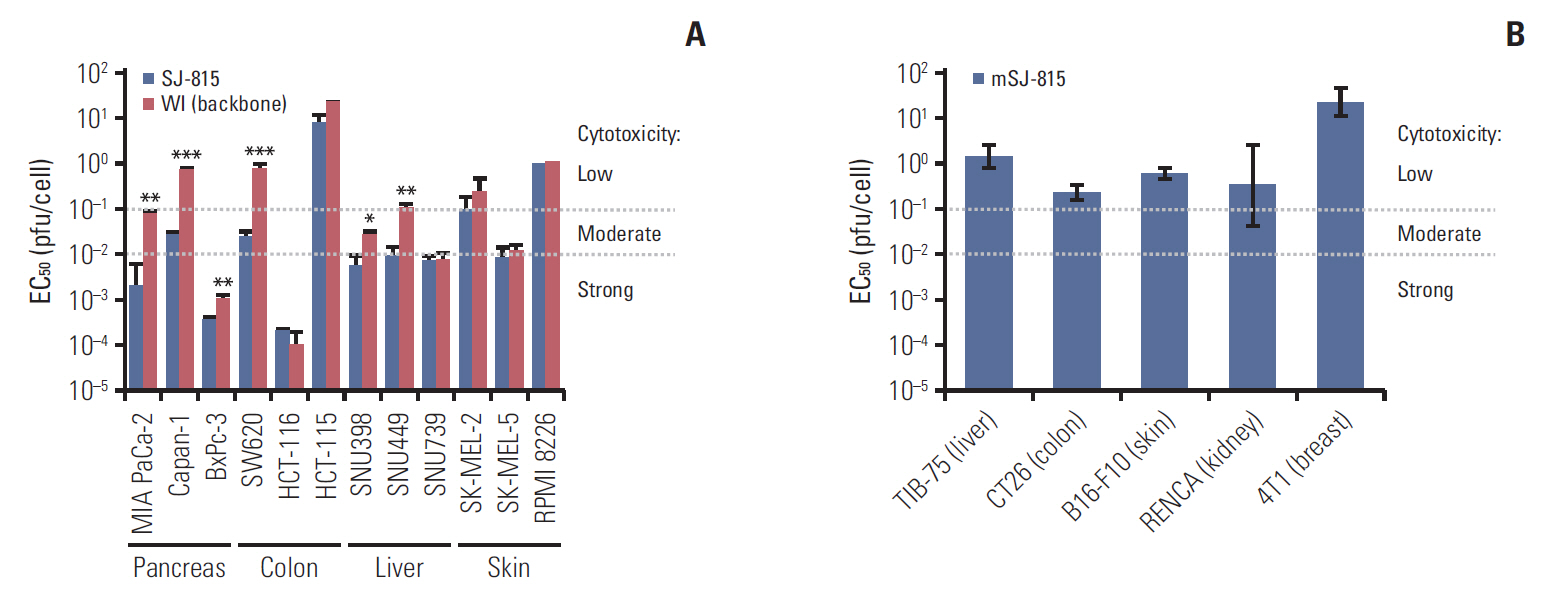
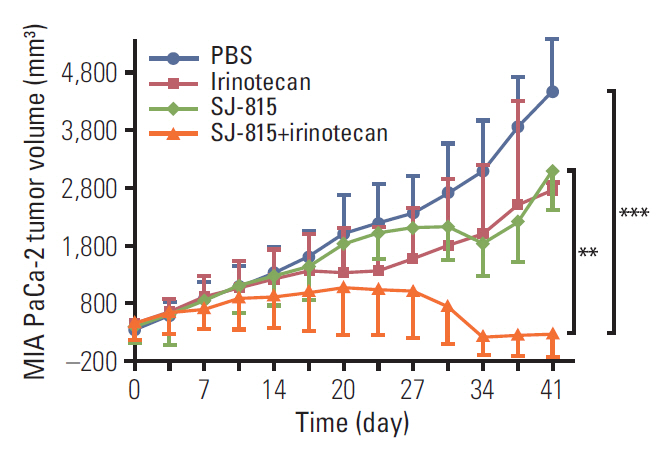
Viral replication and dissemination of SJ-815 were measured by plaque assay and comet assay, respectively, and compared to the backbone of SJ-815, a modified Western Reserve virus named WI. Tumor cytotoxicity of SJ-815 (or mSJ-815, which has the murine IFNB1 transgene for mouse cancers) was evaluated using human and mouse cancer cells. Antitumor effects of SJ-815, with/without irinotecan, were evaluated using a human pancreatic cancer-bearing mouse model and a syngeneic melanoma-bearing mouse model. The SN-38/ irinotecan ratios in mouse melanoma tissue 4 days post irinotecan treatment were compared between groups with and without SJ-815 intravenous injection.
Results
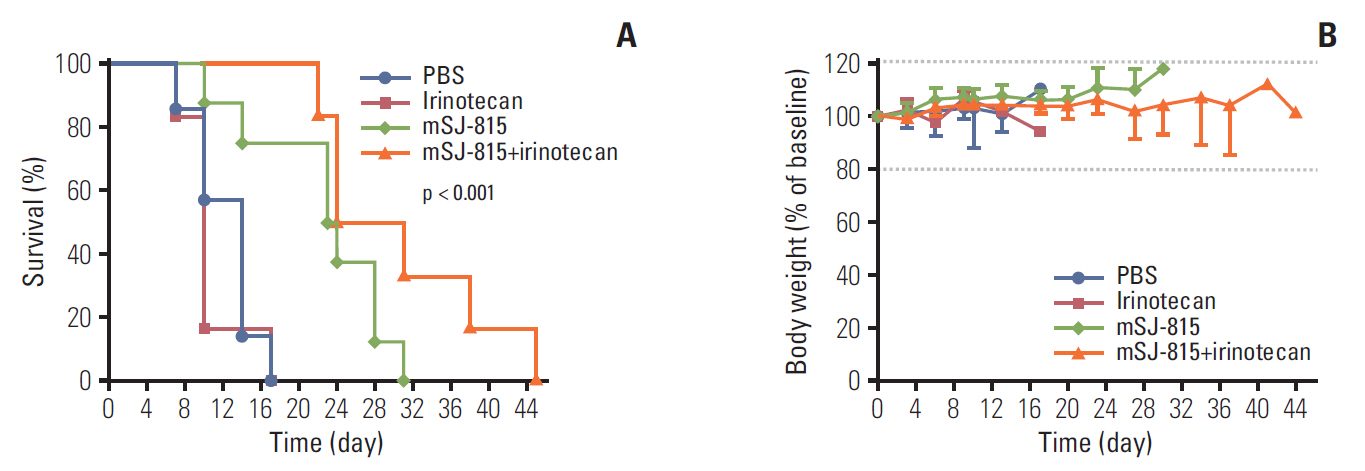
SJ-815 demonstrated significantly lower viral replication and dissemination, but considerably stronger in vitro tumor cytotoxicity than WI. The combination use of SJ-815 plus irinotecan generated substantial tumor regression in the human pancreatic cancer model, and significantly prolonged survival in the melanoma model (hazard ratio, 0.11; 95% confidence interval, 0.02 to 0.50; p=0.013). The tumor SN-38/irinotecan ratios were over 3-fold higher in the group with SJ-815 than those without (p < 0.001).
Conclusion
SJ-815 demonstrates distinct characteristics gained from the inserted IFNB1 and CES2 transgenes. The potent antitumor effects of SJ-815, particularly when combined with irinotecan, against multiple solid tumors make SJ-815 an attractive candidate for further preclinical and clinical studies.
Figure
Reference
-
References
1. Kim MK, Breitbach CJ, Moon A, Heo J, Lee YK, Cho M, et al. Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans. Sci Transl Med. 2013; 5:185ra63.
Article2. Shen Y, Nemunaitis J. Fighting cancer with vaccinia virus: teaching new tricks to an old dog. Mol Ther. 2005; 11:180–95.
Article3. Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008; 9:533–42.
Article4. McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001; 61:8751–7.5. Topolcan O, Holubec L Jr. The role of thymidine kinase in cancer diseases. Expert Opin Med Diagn. 2008; 2:129–41.
Article6. Qin XQ, Tao N, Dergay A, Moy P, Fawell S, Davis A, et al. Interferon-beta gene therapy inhibits tumor formation and causes regression of established tumors in immune-deficient mice. Proc Natl Acad Sci U S A. 1998; 95:14411–6.7. Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le Bon A, Melero I. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res. 2011; 17:2619–27.
Article8. Spaapen RM, Leung MY, Fuertes MB, Kline JP, Zhang L, Zheng Y, et al. Therapeutic activity of high-dose intratumoral IFN-β requires direct effect on the tumor vasculature. J Immunol. 2014; 193:4254–60.
Article9. Kirn DH, Wang Y, Le Boeuf F, Bell J, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007; 4:e353.
Article10. Robertson J, Barr R, Shulman LN, Forte GB, Magrini N. Essential medicines for cancer: WHO recommendations and national priorities. Bull World Health Organ. 2016; 94:735–42.
Article11. Chang JS, Chiu YF, Yu JC, Chen LT, Ch'ang HJ. The role of consolidation chemoradiotherapy in locally advanced pancreatic cancer receiving chemotherapy: an updated systematic review and meta-analysis. Cancer Res Treat. 2018; 50:562–74.
Article12. Cecchin E, Corona G, Masier S, Biason P, Cattarossi G, Frustaci S, et al. Carboxylesterase isoform 2 mRNA expression in peripheral blood mononuclear cells is a predictive marker of the irinotecan to SN38 activation step in colorectal cancer patients. Clin Cancer Res. 2005; 11(19 Pt 1):6901–7.
Article13. Laizure SC, Herring V, Hu Z, Witbrodt K, Parker RB. The role of human carboxylesterases in drug metabolism: have we overlooked their importance? Pharmacotherapy. 2013; 33:210–22.
Article14. Capello M, Lee M, Wang H, Babel I, Katz MH, Fleming JB, et al. Carboxylesterase 2 as a determinant of response to irinotecan and neoadjuvant FOLFIRINOX therapy in pancreatic ductal adenocarcinoma. J Natl Cancer Inst. 2015; 107:djv132.
Article15. Wadkins RM, Hyatt JL, Yoon KJ, Morton CL, Lee RE, Damodaran K, et al. Discovery of novel selective inhibitors of human intestinal carboxylesterase for the amelioration of irinotecan-induced diarrhea: synthesis, quantitative structure-activity relationship analysis, and biological activity. Mol Pharmacol. 2004; 65:1336–43.
Article16. Smith GL, Vanderplasschen A, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002; 83(Pt 12):2915–31.
Article17. Bekisz J, Baron S, Balinsky C, Morrow A, Zoon KC. Antiproliferative properties of type I and type II interferon. Pharmaceuticals (Basel). 2010; 3:994–1015.
Article18. Blasco R, Sisler JR, Moss B. Dissociation of progeny vaccinia virus from the cell membrane is regulated by a viral envelope glycoprotein: effect of a point mutation in the lectin homology domain of the A34R gene. J Virol. 1993; 67:3319–25.
Article19. Sampath P, Li J, Hou W, Chen H, Bartlett DL, Thorne SH. Crosstalk between immune cell and oncolytic vaccinia therapy enhances tumor trafficking and antitumor effects. Mol Ther. 2013; 21:620–8.
Article20. Khan S, Ahmad A, Guo W, Wang YF, Abu-Qare A, Ahmad I. A simple and sensitive LC/MS/MS assay for 7-ethyl-10-hydroxycamptothecin (SN-38) in mouse plasma and tissues: application to pharmacokinetic study of liposome entrapped SN-38 (LE-SN38). J Pharm Biomed Anal. 2005; 37:135–42.
Article21. Vanderplasschen A, Hollinshead M, Smith GL. Antibodies against vaccinia virus do not neutralize extracellular enveloped virus but prevent virus release from infected cells and comet formation. J Gen Virol. 1997; 78(Pt 8):2041–8.
Article22. Potts KG, Irwin CR, Favis NA, Pink DB, Vincent KM, Lewis JD, et al. Deletion of F4L (ribonucleotide reductase) in vaccinia virus produces a selective oncolytic virus and promotes antitumor immunity with superior safety in bladder cancer models. EMBO Mol Med. 2017; 9:638–54.23. Leib DA, Machalek MA, Williams BR, Silverman RH, Virgin HW. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc Natl Acad Sci U S A. 2000; 97:6097–101.
Article24. Vaha-Koskela M, Hinkkanen A. Tumor restrictions to oncolytic virus. Biomedicines. 2014; 2:163–94.
Article25. Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for anti-tumor CD8+ T cell responses through CD8α+ dendritic cells. J Exp Med. 2011; 208:2005–16.
Article26. Yang X, Zhang X, Fu ML, Weichselbaum RR, Gajewski TF, Guo Y, et al. Targeting the tumor microenvironment with interferon-β bridges innate and adaptive immune responses. Cancer Cell. 2014; 25:37–48.
Article27. Gujar S, Pol JG, Kroemer G. Heating it up: oncolytic viruses make tumors 'hot' and suitable for checkpoint blockade immunotherapies. Oncoimmunology. 2018; 7:e1442169.
Article28. Patel MR, Jacobson BA, Ji Y, Drees J, Tang S, Xiong K, et al. Vesicular stomatitis virus expressing interferon-β is oncolytic and promotes antitumor immune responses in a syngeneic murine model of non-small cell lung cancer. Oncotarget. 2015; 6:33165–77.
Article29. Hsieh YT, Lin HP, Chen BM, Huang PT, Roffler SR. Effect of cellular location of human carboxylesterase 2 on CPT-11 hydrolysis and anticancer activity. PLoS One. 2015; 10:e0141088.
Article30. Chu C, Abbara C, Tandia M, Polrot M, Gonin P, Farinotti R, et al. Cetuximab increases concentrations of irinotecan and of its active metabolite SN-38 in plasma and tumour of human colorectal carcinoma-bearing mice. Fundam Clin Pharmacol. 2014; 28:652–60.
Article31. Sharkey RM, McBride WJ, Cardillo TM, Govindan SV, Wang Y, Rossi EA, et al. Enhanced delivery of SN-38 to human tumor xenografts with an anti-Trop-2-SN-38 antibody conjugate (Sacituzumab Govitecan). Clin Cancer Res. 2015; 21:5131–8.
Article32. Adkins CE, Nounou MI, Hye T, Mohammad AS, Terrell-Hall T, Mohan NK, et al. NKTR-102 Efficacy versus irinotecan in a mouse model of brain metastases of breast cancer. BMC Cancer. 2015; 15:685.
Article33. Cho E, Ryu EJ, Jiang F, Jeon UB, Cho M, Kim CH, et al. Preclinical safety evaluation of hepatic arterial infusion of oncolytic poxvirus. Drug Des Devel Ther. 2018; 12:2467–74.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Targeted Inhibition of p21 Promotes the Growth of Breast Cancer Cells and Impairs the Tumor-Killing Effect of the Vaccinia Virus
- Effect of aldosterone on the amplification of oncolytic vaccinia virus in human cancer lines
- Effective Antitumor Activity of a Recombinant Vaccinia Virus Expressing Murine Interleukin 4
- Vaccinia of the Lids
- An Experimental Study of the Effect of Simultaneous Vaccination with B. C. G. and Smallpox Vaccine -Part 2. Experiments in the innunized aminals and the rabbit kidney fibroblast monolayer cell culture