Int J Stem Cells.
2020 Mar;13(1):116-126. 10.15283/ijsc19094.
Protective Effect of Human Mesenchymal Stem Cells on the Survival of Pancreatic Islets
- Affiliations
-
- 1Experimental Neurology Unit, School of Medicine and Surgery, University of Milano-Bicocca, Monza (MB), Italy
- 2PhD Program in Neuroscience, University of Milano-Bicocca, Monza (MB), Italy
- 3NeuroMi, Milan Center for Neurosciences, Milano, Italy
- 4Department of Biomedical Engineering, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Bergamo, Italy
- 5Department of Management, Information and Production Engineering, University of Bergamo, Dalmine (BG), Italy
- 6Centro Ricerca Tettamanti, Clinica Pediatrica, Università Milano-Bicocca, Monza (MB), Italy
- KMID: 2500571
- DOI: http://doi.org/10.15283/ijsc19094
Abstract
- Background and Objectives
Transplantation of pancreatic islets is an intriguing new therapeutic option to face the worldwide spread problem of Type-I diabetes. Currently, its clinical use is limited by several problems, mainly based on the high number of islets required to restore normoglycaemia and by the low survival of the transplanted tissue. A promising attempt to overcome the limits to such an approach was represented by the use of Mesenchymal Stem Cells (MSC). Despite the encouraging results obtained with murine-derived MSC, little is still known about their protective mechanisms. The aim of the present study was to verify the effectiveness, (besides murine MSC), of clinically relevant human-derived MSC (hMSC) on protecting pancreatic islets, thus also shedding light on the putative differences between MSC of different origin.
Methods and Results
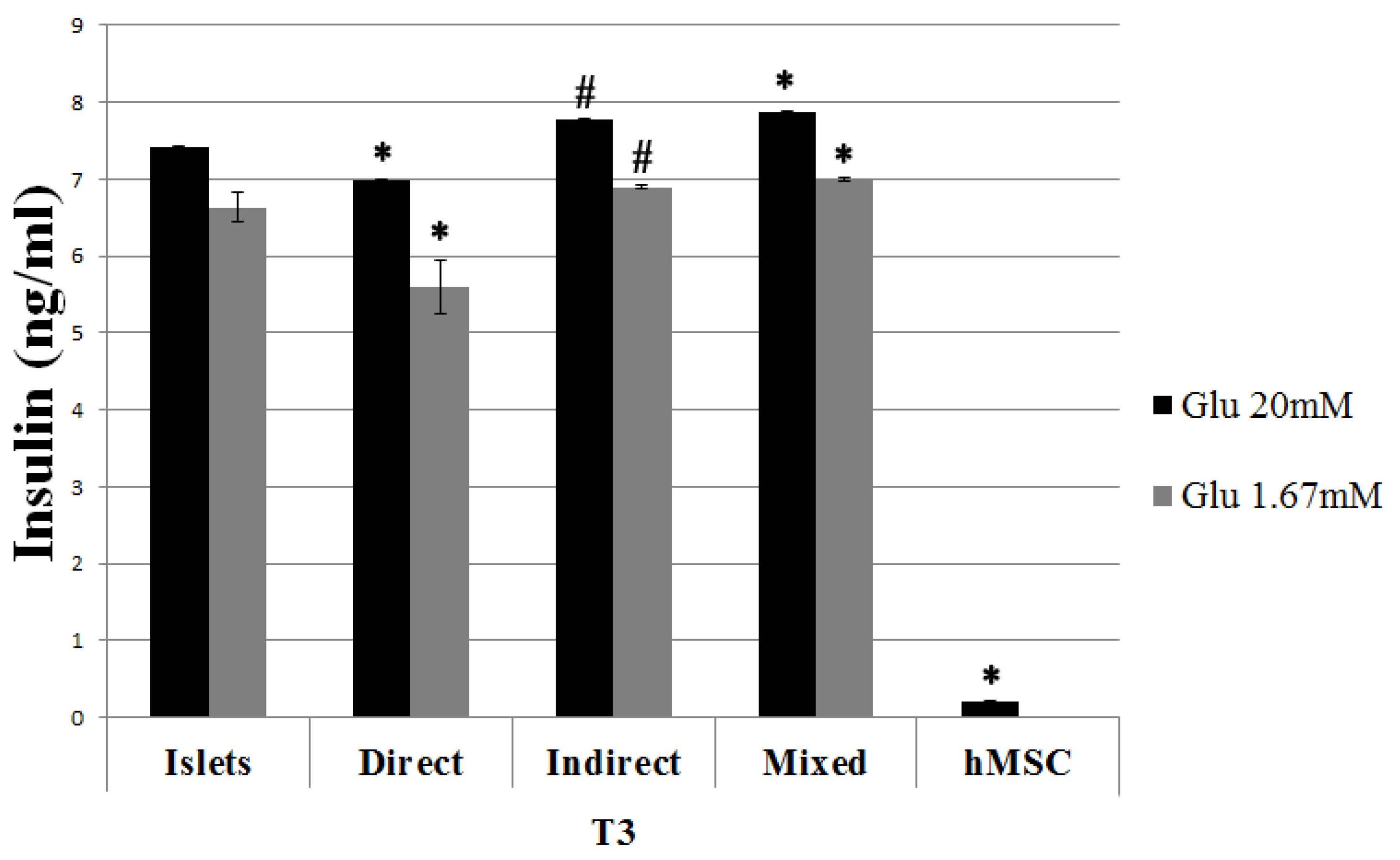
Threefold kinds of co-cultures were therefore in vitro set up (direct, indirect and mixed), to analyze the hMSC effect on pancreatic islet survival and function and to study the putative mechanisms involved. Although in a different way with respect to murine MSC, also human derived cells demonstrated to be effective on protecting pancreatic islet survival. This effect could be due to the release of some trophic factors, such as VEGF and Il-6, and by the reduction of inflammatory cytokine TNF-α.
Conclusions
Therefore, hMSC confirmed their great clinical potential to improve the feasibility of pancreatic islet transplantation therapy against diabetes.
Figure
Reference
-
References
1. Coronel MM, Stabler CL. 2013; Engineering a local microenvironment for pancreatic islet replacement. Curr Opin Biotechnol. 24:900–908. DOI: 10.1016/j.copbio.2013.05.004. PMID: 23769320. PMCID: PMC3783544.
Article2. Johnson PR, Jones KE. 2012; Pancreatic islet transplantation. Semin Pediatr Surg. 21:272–280. DOI: 10.1053/j.sempedsurg.2012.05.012. PMID: 22800980.
Article3. Ezquer F, Ezquer M, Contador D, Ricca M, Simon V, Conget P. 2012; The antidiabetic effect of mesenchymal stem cells is unrelated to their transdifferentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment. Stem Cells. 30:1664–1674. DOI: 10.1002/stem.1132. PMID: 22644660.
Article4. Mujica-Mota MA, Patel N, Saliba I. 2018; Hearing loss in type 1 diabetes: are we facing another microvascular disease? A meta-analysis. Int J Pediatr Otorhinolaryngol. 113:38–45. DOI: 10.1016/j.ijporl.2018.07.005. PMID: 30174007.
Article5. Sanlioglu AD, Altunbas HA, Balci MK, Griffith TS, Sanlioglu S. 2013; Clinical utility of insulin and insulin analogs. Islets. 5:67–78. DOI: 10.4161/isl.24590. PMID: 23584214. PMCID: PMC4204021.
Article6. Yeung TY, Seeberger KL, Kin T, Adesida A, Jomha N, Shapiro AM, Korbutt GS. 2012; Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS One. 7:e38189. DOI: 10.1371/journal.pone.0038189. PMID: 22666480. PMCID: PMC3364233.
Article7. Emamaullee JA, Shapiro AM. 2007; Factors influencing the loss of beta-cell mass in islet transplantation. Cell Transplant. 16:1–8. DOI: 10.3727/000000007783464461. PMID: 28880676.
Article8. Chen Y, Shao JZ, Xiang LX, Dong XJ, Zhang GR. 2008; Mesenchymal stem cells: a promising candidate in regenerative medicine. Int J Biochem Cell Biol. 40:815–820. DOI: 10.1016/j.biocel.2008.01.007. PMID: 18295530.
Article9. Uccelli A, Moretta L, Pistoia V. 2008; Mesenchymal stem cells in health and disease. Nat Rev Immunol. 8:726–736. DOI: 10.1038/nri2395. PMID: 19172693.
Article10. Murphy MB, Moncivais K, Caplan AI. 2013; Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 45:e54. DOI: 10.1038/emm.2013.94. PMID: 24232253. PMCID: PMC3849579.
Article11. Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. 2005; Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 106:1755–1761. DOI: 10.1182/blood-2005-04-1496. PMID: 15905186.
Article12. Battiwalla M, Hematti P. 2009; Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy. 11:503–515. DOI: 10.1080/14653240903193806. PMID: 19728189. PMCID: PMC2766085.
Article13. Tchokonte-Nana V, Manda JK. 2018; Early islets and mesenchyme from an injured adult pancreas improve syngeneic engraftments and islet graft function in diabetic rats. Acta Histochem. 120:356–362. DOI: 10.1016/j.acthis.2018.03.008. PMID: 29622345.
Article14. Park KS, Kim YS, Kim JH, Choi B, Kim SH, Tan AH, Lee MS, Lee MK, Kwon CH, Joh JW, Kim SJ, Kim KW. 2010; Trophic molecules derived from human mesenchymal stem cells enhance survival, function, and angiogenesis of isolated islets after transplantation. Transplantation. 89:509–517. DOI: 10.1097/TP.0b013e3181c7dc99. PMID: 20125064.
Article15. Remuzzi A, Cornolti R, Bianchi R, Figliuzzi M, Porretta-Serapiglia C, Oggioni N, Carozzi V, Crippa L, Avezza F, Fiordaliso F, Salio M, Lauria G, Lombardi R, Cavaletti G. 2009; Regression of diabetic complications by islet transplantation in the rat. Diabetologia. 52:2653–2661. DOI: 10.1007/s00125-009-1537-y. PMID: 19789851.
Article16. Salvadè A, Belotti D, Donzelli E, D'Amico G, Gaipa G, Renoldi G, Carini F, Baldoni M, Pogliani E, Tredici G, Biondi A, Biagi E. 2007; GMP-grade preparation of biomimetic scaffolds with osteo-differentiated autologous mesenchymal stromal cells for the treatment of alveolar bone resorption in periodontal disease. Cytotherapy. 9:427–438. DOI: 10.1080/14653240701341995. PMID: 17786604.
Article17. Donzelli E, Lucchini C, Ballarini E, Scuteri A, Carini F, Tredici G, Miloso M. 2011; ERK1 and ERK2 are involved in recruitment and maturation of human mesenchymal stem cells induced to adipogenic differentiation. J Mol Cell Biol. 3:123–131. DOI: 10.1093/jmcb/mjq050. PMID: 21278199.
Article18. Scuteri A, Donzelli E, Rodriguez-Menendez V, Ravasi M, Monfrini M, Bonandrini B, Figliuzzi M, Remuzzi A, Tredici G. 2014; A double mechanism for the mesenchymal stem cells' positive effect on pancreatic islets. PLoS One. 9:e84309. DOI: 10.1371/journal.pone.0084309. PMID: 24416216. PMCID: PMC3885554.
Article19. Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, Feng Y, Gao Q, Chopp M. 2007; Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 27:1684–1691. DOI: 10.1038/sj.jcbfm.9600475. PMID: 17356562. PMCID: PMC2796470.
Article20. Johansson A, Olerud J, Johansson M, Carlsson PO. 2009; Angiostatic factors normally restrict islet endothelial cell proliferation and migration: implications for islet transplantation. Transpl Int. 22:1182–1188. DOI: 10.1111/j.1432-2277.2009.00939.x. PMID: 19891047.
Article21. Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. 2005; Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 105:2214–2219. DOI: 10.1182/blood-2004-07-2921. PMID: 15514012.
Article22. Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. 2005; Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 105:4120–4126. DOI: 10.1182/blood-2004-02-0586. PMID: 15692068.
Article23. Marzorati S, Pileggi A, Ricordi C. 2007; Allogeneic islet transplantation. Expert Opin Biol Ther. 7:1627–1645. DOI: 10.1517/14712598.7.11.1627. PMID: 17961088.
Article24. Monfrini M, Donzelli E, Rodriguez-Menendez V, Ballarini E, Carozzi VA, Chiorazzi A, Meregalli C, Canta A, Oggioni N, Crippa L, Avezza F, Silvani S, Bonandrini B, Figliuzzi M, Remuzzi A, Porretta-Serapiglia C, Bianchi R, Lauria G, Tredici G, Cavaletti G, Scuteri A. 2017; Therapeutic potential of Mesenchymal Stem Cells for the treatment of diabetic peripheral neuropathy. Exp Neurol. 288:75–84. DOI: 10.1016/j.expneurol.2016.11.006. PMID: 27851902.
Article25. Figliuzzi M, Cornolti R, Perico N, Rota C, Morigi M, Remuzzi G, Remuzzi A, Benigni A. 2009; Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant Proc. 41:1797–1800. DOI: 10.1016/j.transproceed.2008.11.015. PMID: 19545731.
Article26. Sakata N, Goto M, Yoshimatsu G, Egawa S, Unno M. 2011; Utility of co-transplanting mesenchymal stem cells in islet transplantation. World J Gastroenterol. 17:5150–5105. DOI: 10.3748/wjg.v17.i47.5150. PMID: 22215938. PMCID: PMC3243880.
Article27. Jung EJ, Kim SC, Wee YM, Kim YH, Choi MY, Jeong SH, Lee J, Lim DG, Han DJ. 2011; Bone marrow-derived mesenchymal stromal cells support rat pancreatic islet survival and insulin secretory function in vitro. Cytotherapy. 13:19–29. DOI: 10.3109/14653249.2010.518608. PMID: 21142900.
Article28. Karaoz E, Ayhan S, Okçu A, Aksoy A, Bayazıt G, Osman Gürol A, Duruksu G. 2011; Bone marrow-derived mesenchymal stem cells co-cultured with pancreatic islets display β cell plasticity. J Tissue Eng Regen Med. 5:491–500. DOI: 10.1002/term.342. PMID: 21604384.
Article29. Zavan B, Giorgi C, Bagnara GP, Vindigni V, Abatangelo G, Cortivo R. 2007; Osteogenic and chondrogenic differentiation: comparison of human and rat bone marrow mesenchymal stem cells cultured into polymeric scaffolds. Eur J Histochem. 51(Suppl 1):1–8. PMID: 17703587.30. Scuteri A, Donzelli E, Foudah D, Caldara C, Redondo J, D'Amico G, Tredici G, Miloso M. 2014; Mesengenic differentiation: comparison of human and rat bone marrow mesenchymal stem cells. Int J Stem Cells. 7:127–134. DOI: 10.15283/ijsc.2014.7.2.127. PMID: 25473450. PMCID: PMC4249895.
Article31. Wang G, Li Y, Wang Y, Dong Y, Wang FS, Ding Y, Kang Y, Xu X. 2014; Roles of the co-culture of human umbilical cord Wharton's jelly-derived mesenchymal stem cells with rat pancreatic cells in the treatment of rats with diabetes mellitus. Exp Ther Med. 8:1389–1396. DOI: 10.3892/etm.2014.1985. PMID: 25289028. PMCID: PMC4186331.
Article32. Hou Y, Song C, Xie WJ, Wei Z, Huang RP, Liu W, Zhang ZL, Shi YB. 2009; Excellent effect of three-dimensional culture condition on pancreatic islets. Diabetes Res Clin Pract. 86:11–15. DOI: 10.1016/j.diabres.2009.07.010. PMID: 19679368.
Article33. Karaoz E, Genç ZS, Demircan PÇ, Aksoy A, Duruksu G. 2010; Protection of rat pancreatic islet function and viability by coculture with rat bone marrow-derived mesenchymal stem cells. Cell Death Dis. 1:e36. DOI: 10.1038/cddis.2010.14. PMID: 21364643. PMCID: PMC3032304.
Article34. Duprez IR, Johansson U, Nilsson B, Korsgren O, Magnusson PU. 2011; Preparatory studies of composite mesenchymal stem cell islets for application in intraportal islet transplantation. Ups J Med Sci. 116:8–17. DOI: 10.3109/03009734.2010.524320. PMID: 21050099. PMCID: PMC3039755.
Article35. Wei Z, Chen N, Guo H, Wang X, Xu F, Ren Q, Lu S, Liu B, Zhang L, Zhao H. 2009; Bone marrow mesenchymal stem cells from leukemia patients inhibit growth and apoptosis in serum-deprived K562 cells. J Exp Clin Cancer Res. 28:141. DOI: 10.1186/1756-9966-28-141. PMID: 19883517. PMCID: PMC2779804.
Article36. Santos Nascimento D, Mosqueira D, Sousa LM, Teixeira M, Filipe M, Resende TP, Araújo AF, Valente M, Almeida J, Martins JP, Santos JM, Bárcia RN, Cruz P, Cruz H, Pinto-do-Ó P. 2014; Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling after myocardial infarction by proangiogenic, antiapoptotic, and endogenous cell-activation mechanisms. Stem Cell Res Ther. 5:5. DOI: 10.1186/scrt394. PMID: 24411922. PMCID: PMC4055157.
Article37. Ichii H, Wang X, Messinger S, Alvarez A, Fraker C, Khan A, Kuroda Y, Inverardi L, Goss JA, Alejandro R, Ricordi C. 2006; Improved human islet isolation using nicotinamide. Am J Transplant. 6:2060–2068. DOI: 10.1111/j.1600-6143.2006.01452.x. PMID: 16827790.
Article38. Hajizadeh-Saffar E, Tahamtani Y, Aghdami N, Azadmanesh K, Habibi-Anbouhi M, Heremans Y, De Leu N, Heimberg H, Ravassard P, Shokrgozar MA, Baharvand H. 2015; Inducible VEGF expression by human embryonic stem cell-derived mesenchymal stromal cells reduces the minimal islet mass required to reverse diabetes. Sci Rep. 5:9322. DOI: 10.1038/srep09322. PMID: 25818803. PMCID: PMC4377549.
Article39. Ren G, Su J, Zhang L, Zhao X, Ling W, L'huillie A, Zhang J, Lu Y, Roberts AI, Ji W, Zhang H, Rabson AB, Shi Y. 2009; Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 27:1954–1962. DOI: 10.1002/stem.118. PMID: 19544427.
Article40. Foudah D, Redondo J, Caldara C, Carini F, Tredici G, Miloso M. 2013; Human mesenchymal stem cells express neuronal markers after osteogenic and adipogenic differentiation. Cell Mol Biol Lett. 18:163–186. DOI: 10.2478/s11658-013-0083-2. PMID: 23430457. PMCID: PMC6275956.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of engineered cell sheets composed of human islets and supporting stem cells enhances the outcome of islet cell transplantation in vitro and in vivo
- Cell Replacement and Regeneration Therapy for Diabetes
- Establishment and Advancement of Pancreatic Organoids
- Umbilical Cord Derived Mesenchymal Stem Cells Useful in Insulin Production - Another Opportunity in Cell Therapy
- Recent Trends and Strategies in Stem Cell Therapy for Alzheimer's Disease