Cancer Res Treat.
2020 Apr;52(2):446-454. 10.4143/crt.2019.261.
Carcinoembryonic Antigen Improves the Performance of MagneticResonance Imaging in the Prediction of Pathologic Response afterNeoadjuvant Chemoradiation for Patients with Rectal Cancer
- Affiliations
-
- 1Department of Radiation Oncology, Internal Medicine
- 2Division of Hematology-Oncology, Internal Medicine
- 3Departments of General Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Departments of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Departments of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2500330
- DOI: http://doi.org/10.4143/crt.2019.261
Abstract
- Purpose
The purpose of this study was to investigate the role of carcinoembryonic antigen (CEA) levels in improving the performance of magnetic resonance imaging (MRI) for the prediction of pathologic response after the neoadjuvant chemoradiation (NCRT) for patients with rectal cancer.
Materials and Methods
We retrospectively reviewed the medical records of 524 rectal cancer patients who underwent NCRT and total mesorectal excision between January 2009 and December 2014. The performances of MRI with or without CEA parameters (initial CEA and CEA dynamics) for prediction of pathologic tumor response grade (pTRG) were compared by receiver-operating characteristic analysis with DeLong’s method. Cox regression was used to identify the independent factors associated to pTRG and disease-free survival (DFS) after NCRT.
Results
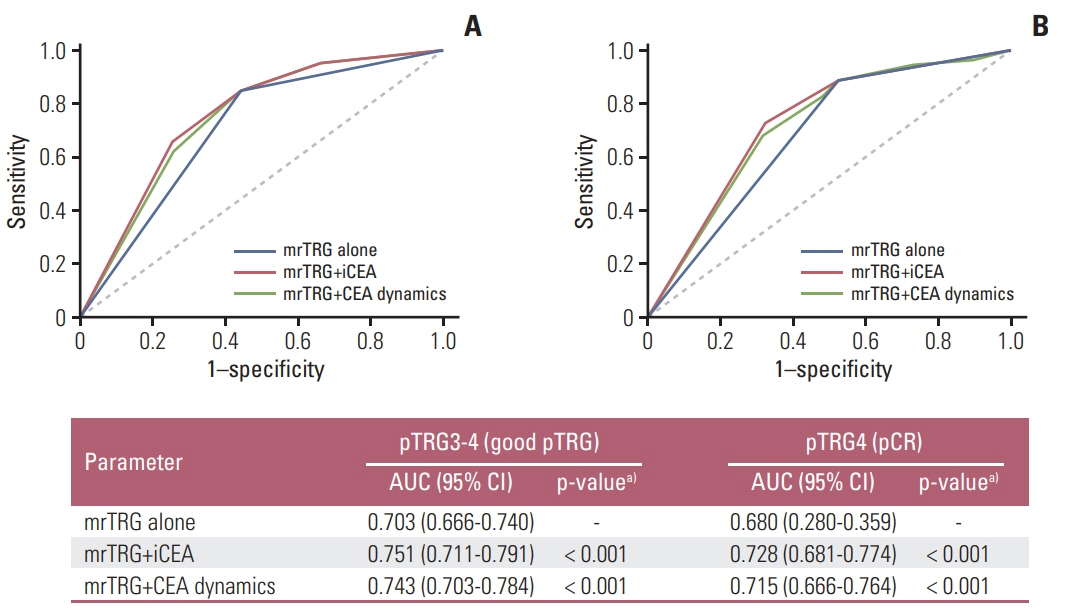
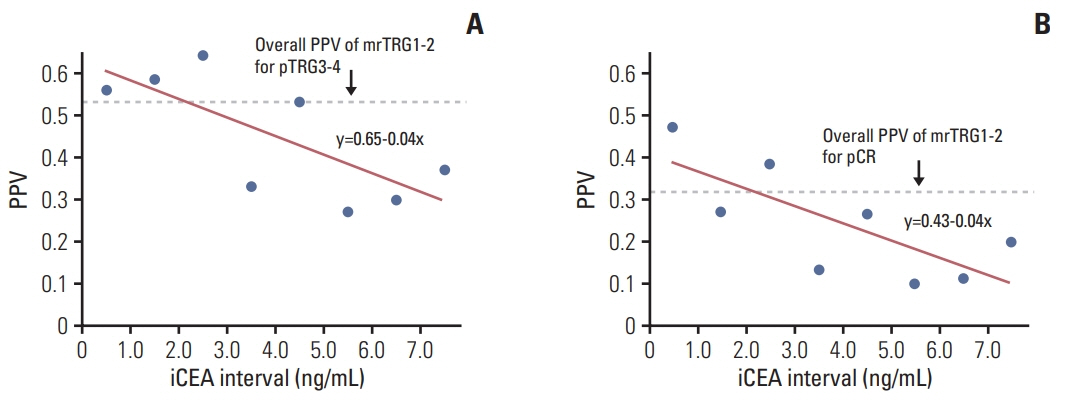
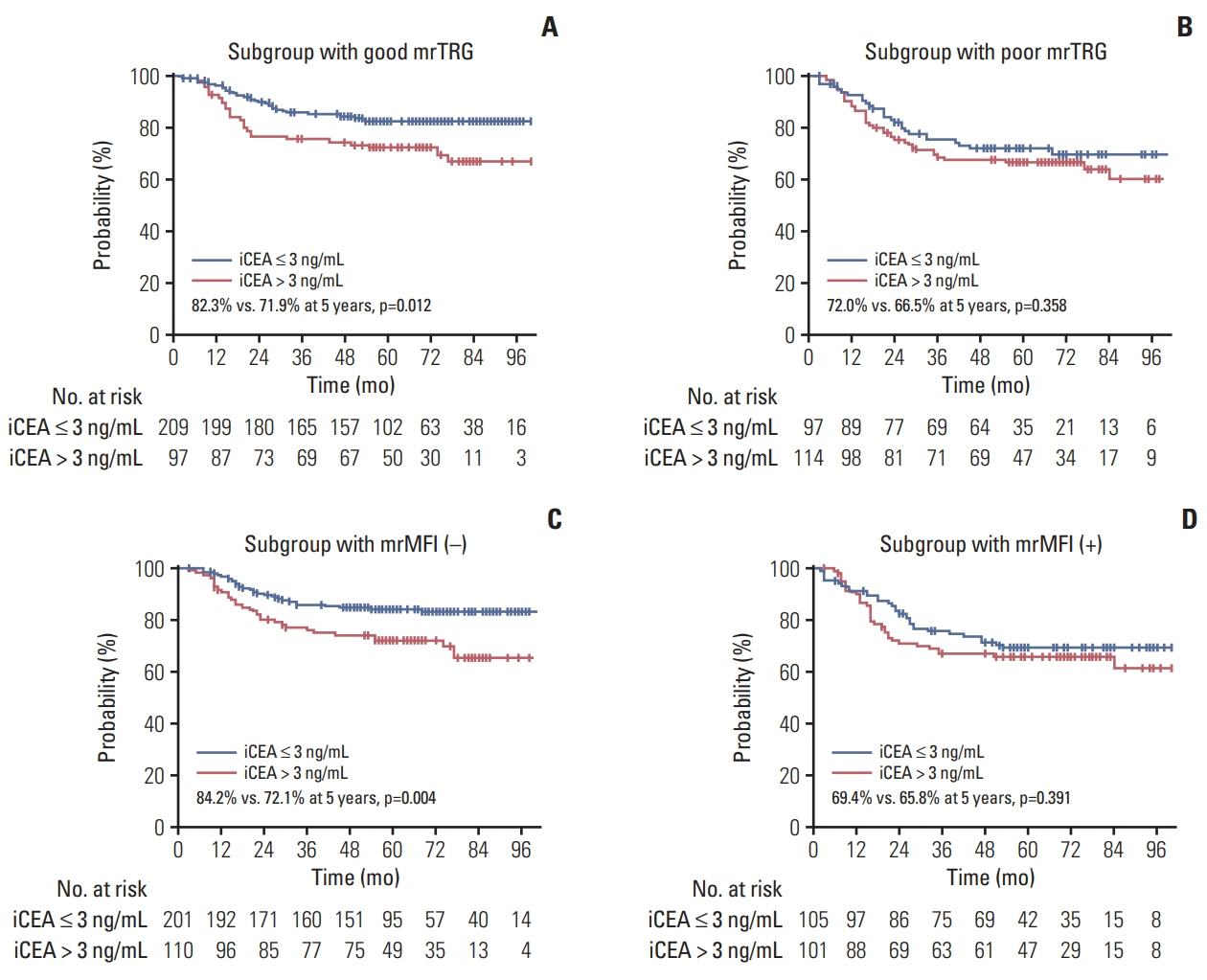
The median follow-up was 64.0 months (range, 3.0 to 113.0 months). On multivariate analysis, poor tumor regression grade on MRI (mrTRG; p < 0.001), initial CEA (p < 0.001) and the mesorectal fascia involvement on MRI before NCRT (mrMFI; p=0.054) showed association with poor pTRG. The mrTRG plus CEA parameters showed significantly improved performances in the prediction of pTRG than mrTRG alone. All of mrTRG, mrMFI, and initial CEA were also identified as independent factors associated with DFS. The initial CEA further discriminated DFS in the subgroups with good mrTRG or that without mrMFI.
Conclusion
The CEA parameters significantly improved the performance of MRI in the prediction of pTRG after NCRT for patients with rectal cancer. The DFS was further discriminated by initial CEA level in the groups with favorable MRI parameters.
Keyword
Figure
Reference
-
References
1. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351:1731–40.
Article2. Song JH, Jeong JU, Lee JH, Kim SH, Cho HM, Um JW, et al. Preoperative chemoradiotherapy versus postoperative chemoradiotherapy for stage II-III resectable rectal cancer: a metaanalysis of randomized controlled trials. Radiat Oncol J. 2017; 35:198–207.
Article3. Rodel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005; 23:8688–96.4. Losi L, Luppi G, Gavioli M, Iachetta F, Bertolini F, D'Amico R, et al. Prognostic value of Dworak grade of regression (GR) in patients with rectal carcinoma treated with preoperative radiochemotherapy. Int J Colorectal Dis. 2006; 21:645–51.
Article5. Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010; 11:835–44.
Article6. Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, GlynneJones R, et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008; 72:99–107.
Article7. Kennedy GD. Hot topics in colorectal surgery. Clin Colon Rectal Surg. 2016; 29:183–4.
Article8. Huang M, Lin J, Yu X, Chen S, Kang L, Deng Y, et al. Erectile and urinary function in men with rectal cancer treated by neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy alone: a randomized trial report. Int J Colorectal Dis. 2016; 31:1349–57.
Article9. Park SY, Choi GS, Park JS, Kim HJ, Ryuk JP, Yun SH. Urinary and erectile function in men after total mesorectal excision by laparoscopic or robot-assisted methods for the treatment of rectal cancer: a case-matched comparison. World J Surg. 2014; 38:1834–42.
Article10. van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018; 391:2537–45.11. Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-andwait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017; 2:501–13.
Article12. Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint AS, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016; 17:174–83.
Article13. Kim JH. Controversial issues in radiotherapy for rectal cancer: a systematic review. Radiat Oncol J. 2017; 35:295–305.
Article14. Goodman KA. Definitive chemoradiotherapy ("Watch-andWait" approach). Semin Radiat Oncol. 2016; 26:205–10.
Article15. Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011; 29:3753–60.
Article16. Patel UB, Brown G, Rutten H, West N, Sebag-Montefiore D, Glynne-Jones R, et al. Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann Surg Oncol. 2012; 19:2842–52.
Article17. Lee JH, Kim DY, Kim SH, Cho HM, Shim BY, Kim TH, et al. Carcinoembryonic antigen has prognostic value for tumor downstaging and recurrence in rectal cancer after preoperative chemoradiotherapy and curative surgery: a multi-institutional and case-matched control study of KROG 14-12. Radiother Oncol. 2015; 116:202–8.
Article18. Kim CW, Yu CS, Yang SS, Kim KH, Yoon YS, Yoon SN, et al. Clinical significance of pre- to post-chemoradiotherapy s-CEA reduction ratio in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol. 2011; 18:3271–7.
Article19. Chung MJ, Nam TK, Jeong JU, Kim SH, Kim K, Jang HS, et al. Can serum dynamics of carcinoembryonic antigen level during neoadjuvant chemoradiotherapy in rectal cancer predict tumor response and recurrence? A multi-institutional retrospective study. Int J Colorectal Dis. 2016; 31:1595–601.
Article20. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–4.
Article21. Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997; 12:19–23.
Article22. Song I, Kim SH, Lee SJ, Choi JY, Kim MJ, Rhim H. Value of diffusion-weighted imaging in the detection of viable tumour after neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer: comparison with T2 weighted and PET/CT imaging. Br J Radiol. 2012; 85:577–86.23. Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000; 45:23–41.
Article24. Bulens P, Couwenberg A, Haustermans K, Debucquoy A, Vandecaveye V, Philippens M, et al. Development and validation of an MRI-based model to predict response to chemoradiotherapy for rectal cancer. Radiother Oncol. 2018; 126:437–42.
Article25. Lambrecht M, Vandecaveye V, De Keyzer F, Roels S, Penninckx F, Van Cutsem E, et al. Value of diffusion-weighted magnetic resonance imaging for prediction and early assessment of response to neoadjuvant radiochemotherapy in rectal cancer: preliminary results. Int J Radiat Oncol Biol Phys. 2012; 82:863–70.
Article26. Barbaro B, Vitale R, Valentini V, Illuminati S, Vecchio FM, Rizzo G, et al. Diffusion-weighted magnetic resonance imaging in monitoring rectal cancer response to neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012; 83:594–9.
Article27. Joye I, Deroose CM, Vandecaveye V, Haustermans K. The role of diffusion-weighted MRI and (18)F-FDG PET/CT in the prediction of pathologic complete response after radiochemotherapy for rectal cancer: a systematic review. Radiother Oncol. 2014; 113:158–65.
Article28. Lee SJ, Kim JG, Lee SW, Chae YS, Kang BW, Lee YJ, et al. Clinical implications of initial FDG-PET/CT in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Cancer Chemother Pharmacol. 2013; 71:1201–7.
Article29. Hindie E, Hennequin C, Moretti JL. Predicting response to chemoradiotherapy in rectal and oesophageal cancer with 18FFDG: prognostic value and possible role in patient management. Eur J Nucl Med Mol Imaging. 2007; 34:1576–82.
Article30. Leccisotti L, Gambacorta MA, de Waure C, Stefanelli A, Barbaro B, Vecchio FM, et al. The predictive value of 18F-FDG PET/CT for assessing pathological response and survival in locally advanced rectal cancer after neoadjuvant radiochemotherapy. Eur J Nucl Med Mol Imaging. 2015; 42:657–66.
Article31. Murcia Durendez MJ, Frutos Esteban L, Lujan J, Frutos MD, Valero G, Navarro Fernandez JL, et al. The value of 18F-FDG PET/CT for assessing the response to neoadjuvant therapy in locally advanced rectal cancer. Eur J Nucl Med Mol Imaging. 2013; 40:91–7.
Article32. Koc M, Kaya GC, Demir Y, Surucu E, Sarioglu S, Obuz F, et al. The value of liver-based standardized uptake value and other quantitative 18F-FDG PET-CT parameters in neoadjuvant therapy response in patients with locally advanced rectal cancer: correlation with histopathology. Nucl Med Commun. 2015; 36:898–907.33. Aiba T, Uehara K, Nihashi T, Tsuzuki T, Yatsuya H, Yoshioka Y, et al. MRI and FDG-PET for assessment of response to neoadjuvant chemotherapy in locally advanced rectal cancer. Ann Surg Oncol. 2014; 21:1801–8.
Article34. Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014; 32:34–43.
Article35. Kim H, Myoung S, Koom WS, Kim NK, Kim MJ, Ahn JB, et al. MRI risk stratification for tumor relapse in rectal cancer achieving pathological complete remission after neoadjuvant chemoradiation therapy and curative resection. PLoS One. 2016; 11:e0146235.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Significance of Serum Carcinoembryonic Antigen Level in Rectal Cancer Patients Who Underwent Preoperative Chemoradiotherapy
- Obesity as an independent predictive factor for pathologic complete response after neoadjuvant chemoradiation in rectal cancer
- New Perspectives on Predictive Biomarkers of Tumor Response and Their Clinical Application in Preoperative Chemoradiation Therapy for Rectal Cancer
- How to Achieve a Higher Pathologic Complete Response in Patients With Locally Advanced Rectal Cancer Who Receive Preoperative Chemoradiation Therapy
- Differences in Serum CEA Level between Colon and Rectal Cancer