Cancer Res Treat.
2020 Apr;52(2):406-418. 10.4143/crt.2019.296.
Apatinib Combined with Local Irradiation Leads to Systemic TumorControl via Reversal of Immunosuppressive Tumor Microenvironmentin Lung Cancer
- Affiliations
-
- 1Department of Oncology, The Aliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang, China
- 2Department of Clinical Medicine, Xuzhou Medical University, Xuzhou, China
- 3Department of Clinical Pharmacology, The Aliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang, China
- 4Medical Imaging Faculty of Xuzhou Medical University, Xuzhou, China
- KMID: 2500326
- DOI: http://doi.org/10.4143/crt.2019.296
Abstract
- Purpose
This study aimed to investigate the potential systemic antitumor effects of stereotactic ablative radiotherapy (SABR) and apatinib (a novel vascular endothelial growth factor receptor 2 inhibitor) via reversing the immunosuppressive tumor microenvironment for lung carcinoma.
Materials and Methods
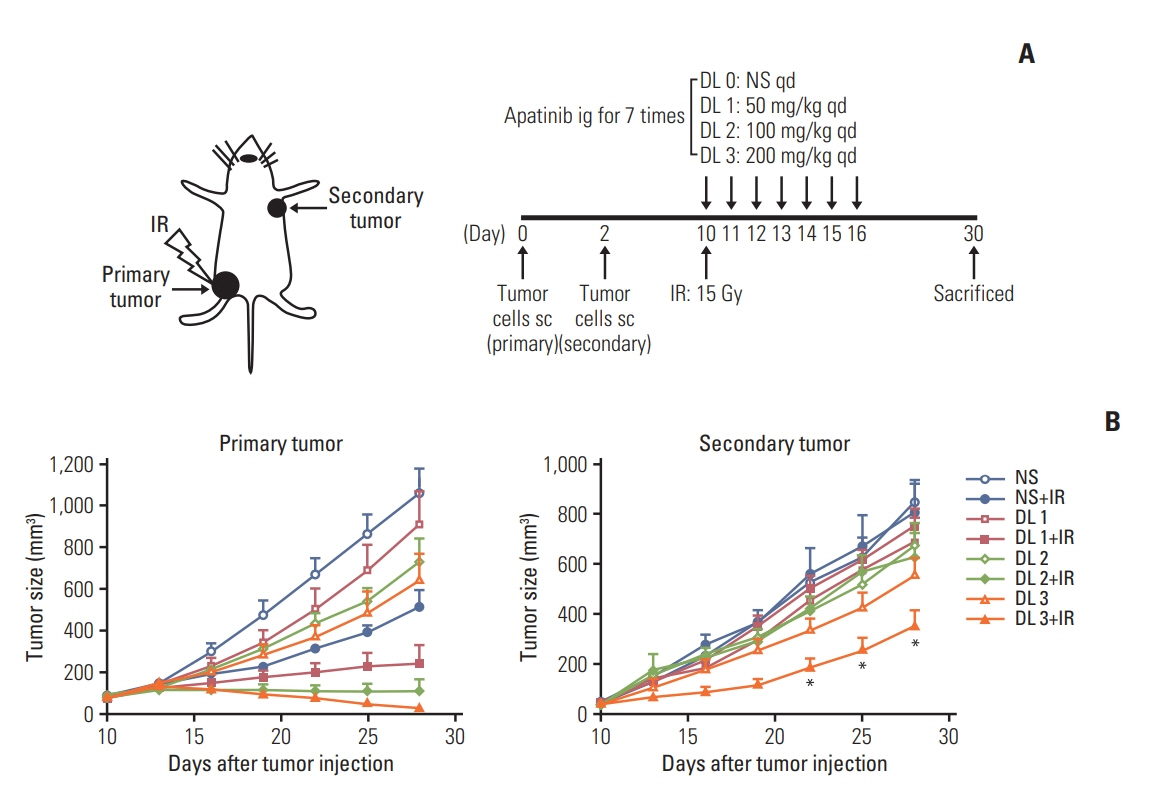
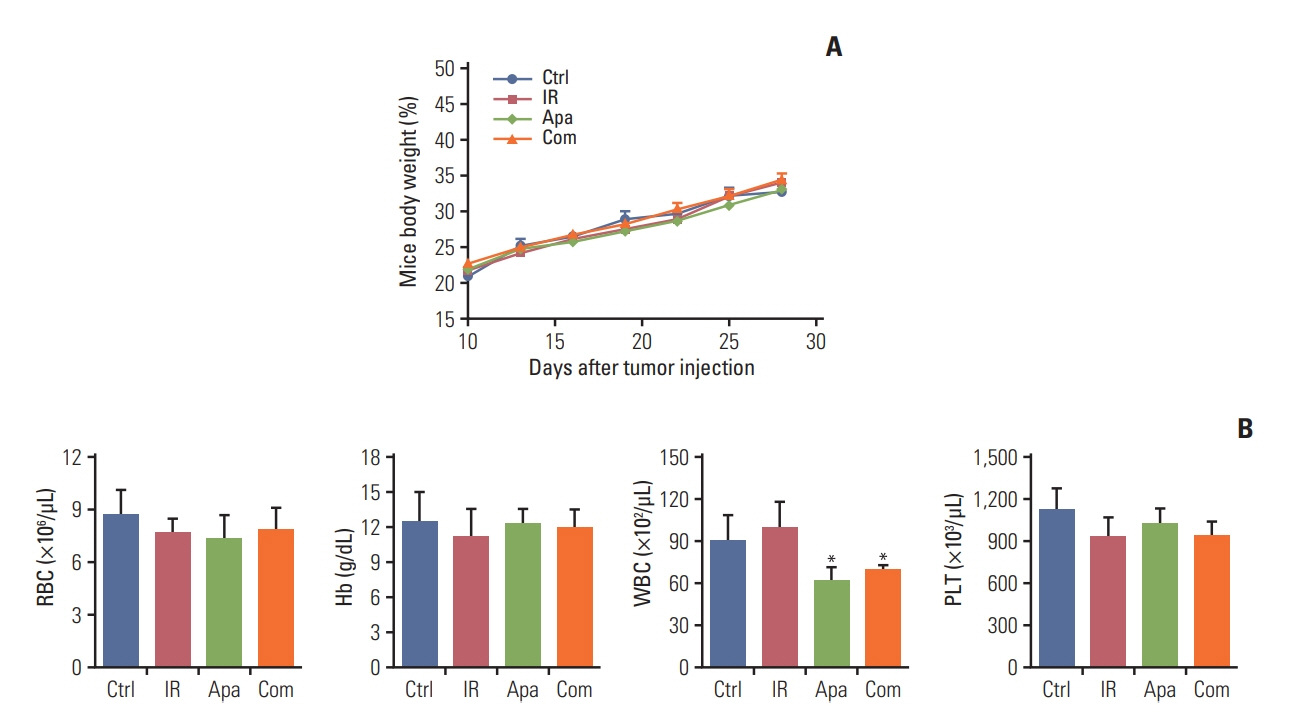
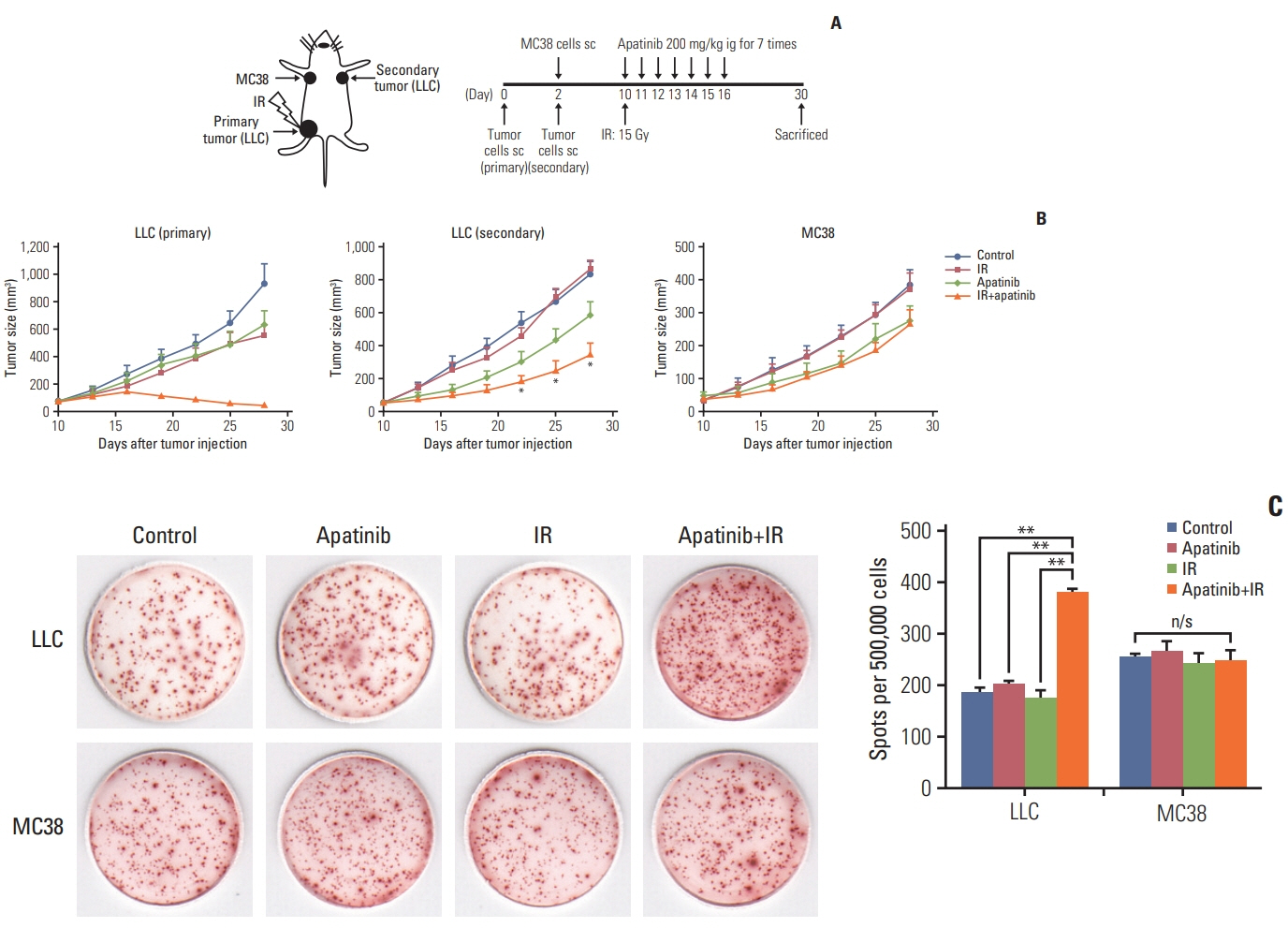
Lewis lung cancer cells were injected into C57BL/6 mice in the left hindlimb (primary tumor; irradiated) and in the right flank (secondary tumor; nonirradiated). When both tumors grew to the touchable size, mice were randomly divided into eight treatment groups. These groups received normal saline or three distinct doses of apatinib (50 mg/kg, 150 mg/kg, and 200 mg/kg) daily for 7 days, in combination with a single dose of 15 Gy radiotherapy or not to the primary tumor. The further tumor growth/regression of mice were followed and observed.
Results
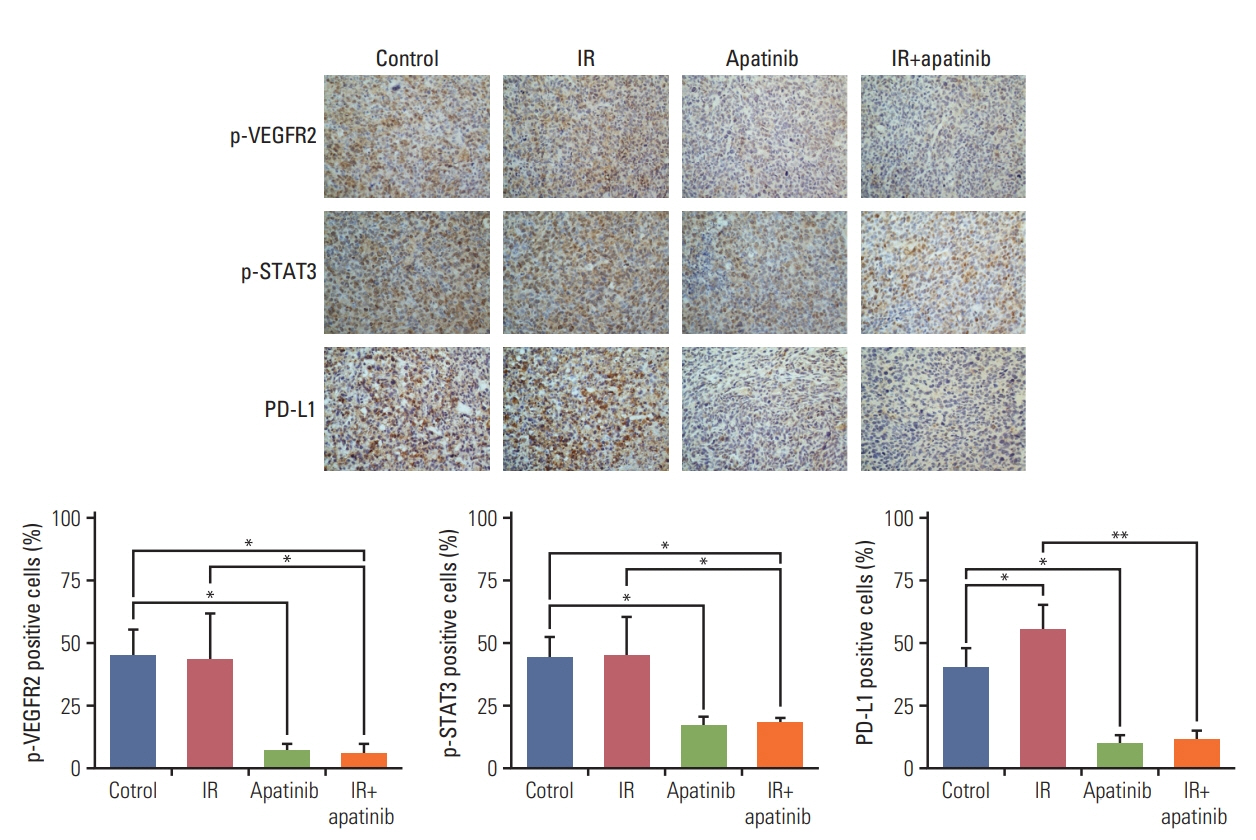
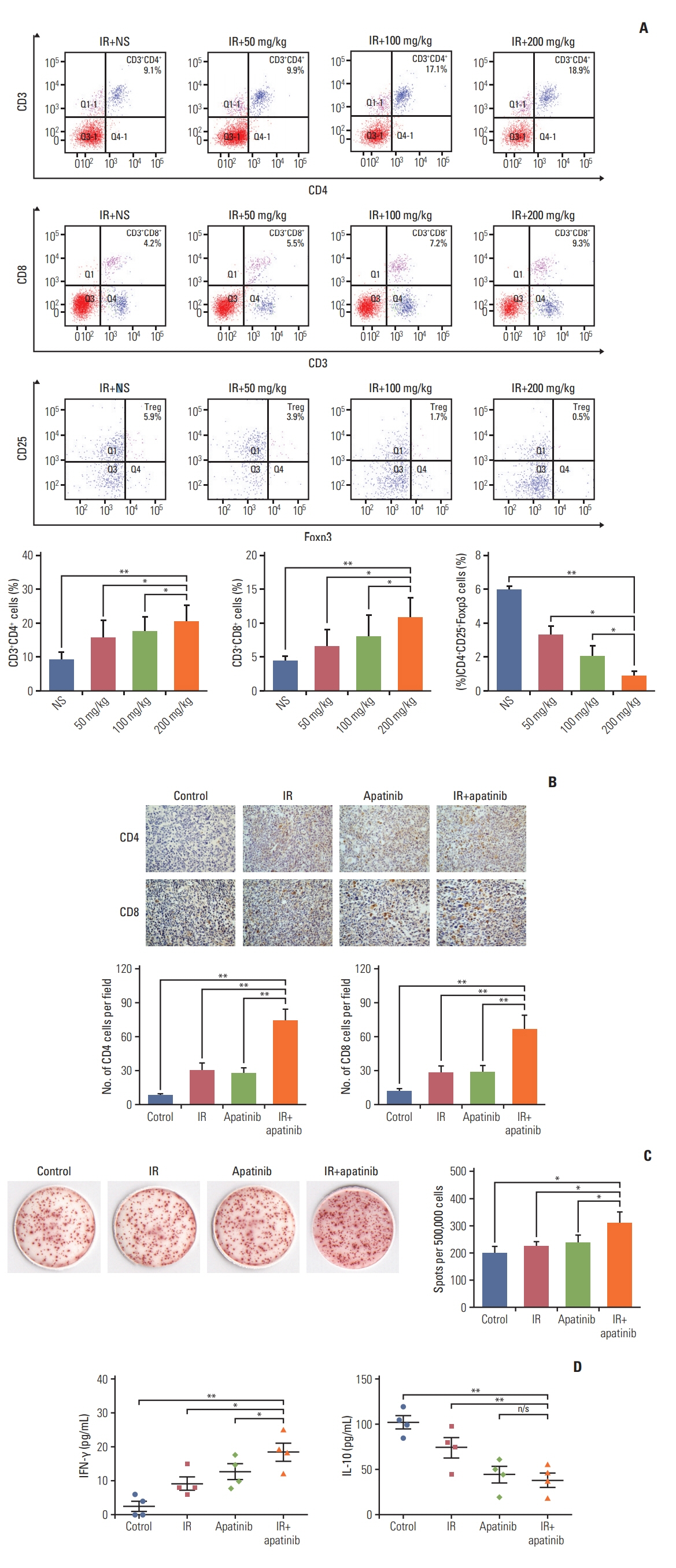
For the single 15 Gy modality, tumor growth delay could only be observed at the primary tumor. When combining SABR and apatinib 200 mg/kg, significant retardation of both primary and secondary tumor growth could be observed, indicated an abscopal effect was induced. Mechanism analysis suggested that programmed death-ligand 1 expression increased with SABR was counteract by additional apatinib therapy. Furthermore, when apatinib was combined with SABR, the composition of immune cells could be changed. More importantly, this two-pronged approach evoked tumor antigen–specific immune responses and the mice were resistant to another tumor rechallenge, finally, long-term survival was improved.
Conclusion
Our results suggested that the tumor microenvironment could be managed with apatinib, which was effective in eliciting an abscopal effect induced by SABR.
Figure
Reference
-
References
1. Jaffray DA. Image-guided radiotherapy: from current concept to future perspectives. Nat Rev Clin Oncol. 2012; 9:688–99.
Article2. Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin. 2017; 67:65–85.
Article3. Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012; 366:925–31.
Article4. Siva S, Callahan J, MacManus MP, Martin O, Hicks RJ, Ball DL. Abscopal [corrected] effects after conventional and stereotactic lung irradiation of non-small-cell lung cancer. J Thorac Oncol. 2013; 8:e71.5. Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013; 85:293–5.
Article6. Wersäll PJ, Blomgren H, Pisa P, Lax I, Kalkner KM, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 2006; 45:493–7.
Article7. Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012; 12:298–306.
Article8. Son CH, Bae JH, Shin DY, Lee HR, Jo WS, Yang K, et al. Combination effect of regulatory T-cell depletion and ionizing radiation in mouse models of lung and colon cancer. Int J Radiat Oncol Biol Phys. 2015; 92:390–8.
Article9. Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, et al. Local radiotherapy and granulocyte- macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015; 16:795–803.10. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014; 124:687–95.
Article11. Park SS, Dong H, Liu X, Harrington SM, Krco CJ, Grams MP, et al. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol Res. 2015; 3:610–9.
Article12. Tian S, Quan H, Xie C, Guo H, Lu F, Xu Y, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011; 102:1374–80.
Article13. Li J, Zhao X, Chen L, Guo H, Lv F, Jia K, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer. 2010; 10:529.
Article14. Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, double- blind, placebo-controlled phase iii trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016; 34:1448–54.15. Zhang L, Shi M, Huang C, Liu X, Xiong JP, Chen G, et al. A phase II, multicenter, placebo-controlled trial of apatinib in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after two previous treatment regimens. J Clin Oncol. 2012; 30(15 Suppl):7548.
Article16. Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J, et al. Control of the immune response by pro-angiogenic factors. Front Oncol. 2014; 4:70.
Article17. Jiang XD, Dai P, Wu J, Song DA, Yu JM. Inhibitory effect of radiotherapy combined with weekly recombinant human endostatin on the human pulmonary adenocarcinoma A549 xenografts in nude mice. Lung Cancer. 2011; 72:165–71.
Article18. Zheng B, Ren T, Huang Y, Guo W. Apatinib inhibits migration and invasion as well as PD-L1 expression in osteosarcoma by targeting STAT3. Biochem Biophys Res Commun. 2018; 495:1695–701.
Article19. Qiu MJ, He XX, Bi NR, Wang MM, Xiong ZF, Yang SL. Effects of liver-targeted drugs on expression of immune-related proteins in hepatocellular carcinoma cells. Clin Chim Acta. 2018; 485:103–5.
Article20. Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol. 2018; 11:104.
Article21. Peng S, Zhang Y, Peng H, Ke Z, Xu L, Su T, et al. Intracellular autocrine VEGF signaling promotes EBDC cell proliferation, which can be inhibited by Apatinib. Cancer Lett. 2016; 373:193–202.
Article22. Liu K, Ren T, Huang Y, Sun K, Bao X, Wang S, et al. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/ BCL-2 signaling in osteosarcoma. Cell Death Dis. 2017; 8:e3015.23. Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018; 48:434–52.
Article24. Yu H, Jove R. The STATs of cancer: new molecular targets come of age. Nat Rev Cancer. 2004; 4:97–105.25. Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016; 27:409–16.
Article26. Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013; 73:539–49.
Article27. Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009; 69:2514–22.
Article28. Ziogas AC, Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, Terpos E, et al. VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int J Cancer. 2012; 130:857–64.
Article29. Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017; 544:250–4.
Article30. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009; 15:5379–88.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Local Irradiation in Prevention and Reversal of Acute Rejection of Transplanted Kidney with High-dose Steroid Pulse
- Comparative evaluation of photobiomodulation therapy at 660 and 810 nm wavelengths on the soft tissue local anesthesia reversal in pediatric dentistry: an in-vivo study
- Recurrent Herpes Zoster During the Treatment of systemic Lupus Erythematosus With Immunosuppressive Drugs
- Decreasing systemic inflammation after TIPS: Still hope for the liver: Reply to correspondence on “Insertion of a transjugular intrahepatic portosystemic shunt leads to sustained reversal of systemic inflammation in patients with decompensated liver cirrhosis”
- TIPS insertion and systemic inflammation: Is it ever too late to lower portal pressure? Correspondence to editorial on “Insertion of a transjugular intrahepatic portosystemic shunt leads to sustained reversal of systemic inflammation in patients with decompensated liver cirrhosis”