Cancer Res Treat.
2020 Apr;52(2):388-395. 10.4143/crt.2019.213.
Displacement of Surgical Clips in Patients with Human AcellularDermal Matrix in the Excision Cavity during Whole Breast IrradiationFollowing Breast-Conserving Surgery
- Affiliations
-
- 1Departments of Radiation Oncology, Ewha Womans University College of Medicine, Seoul, Korea
- 2Departments of Surgery, Ewha Womans University College of Medicine, Seoul, Korea
- KMID: 2500324
- DOI: http://doi.org/10.4143/crt.2019.213
Abstract
- Purpose
The purpose of this study was to investigate the displacement of surgical clips in the excision cavity during whole breast irradiation following breast-conserving surgery (BCS) with or without acellular dermal matrix (ADM) insertion, and to analyze clinicopathologic factors associated with the displacement of surgical clips.
Materials and Methods
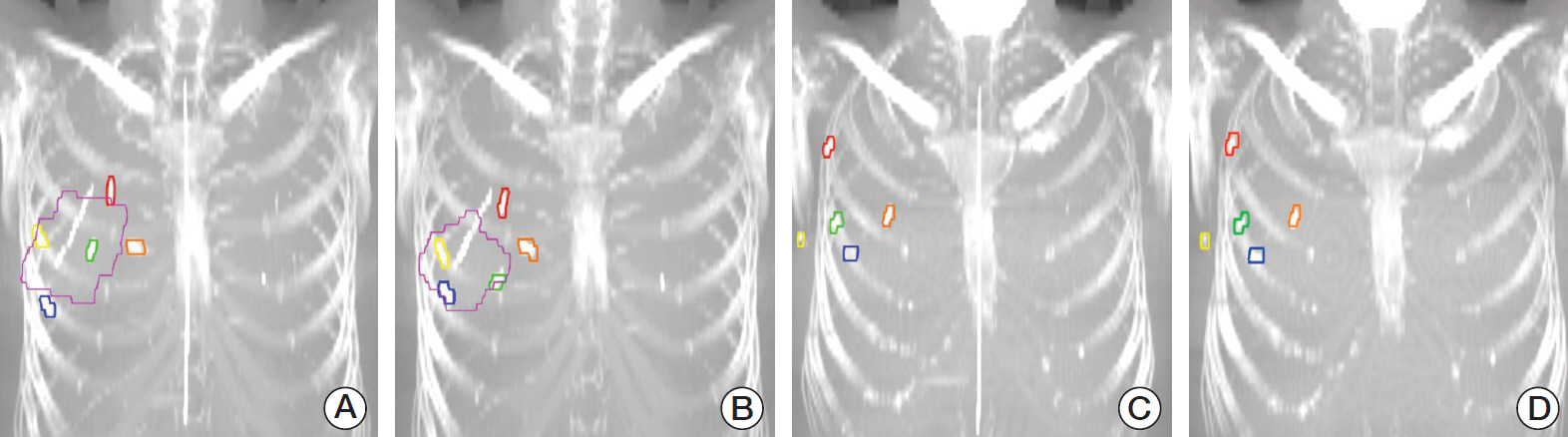
From 2016 to 2017, 100 consecutive breast cancer patients who underwent BCS with the placement of surgical clips (superior, inferior, medial, lateral, and deep sides) in the tumor bed were included in this study. All patients took first planning computed tomography (CT) scan (CT 1) before whole breast irradiation and second CT scan (CT 2) before boost irradiation. Between two sets of planning CT, the displacement of surgical clips was calculated from the !X (lateral–medial), !Y (anterior–posterior), !Z (superior–inferior), and threedimensional (3D) directions. Patients were divided into two groups according to the breast volume replacement with ADM: group A with ADM and group B without ADM.
Results
The means and 1 standard deviations of 3D displacement for superior, inferior, medial, lateral and deep clips were 5.2±2.9, 5.2±3.2, 5.6±4.5, 5.6±4.3, and 4.9±4.9 mm in entire cohort (n=100); 5.6±2.6, 6.0±3.5, 6.7±5.8, 6.7±5.7, and 6.1±7.4 mm in group A (n=38); 4.9±3.1, 4.8±3.0, 5.0±3.5, 5.0±2.9, and 4.3±2.8 mm in group B (n=62), respectively. The 3D displacements of group A were longer than those of group B, but only significant difference was observed in lateral clip (p=0.047).
Conclusion
This study demonstrated displacement of surgical clips during whole breast irradiation in patients with ADM insertion. For patients who had breast volume replacement using ADM, adaptive boost planning should be considered.
Keyword
Figure
Reference
-
References
1. Bartelink H, Horiot JC, Poortmans P, Struikmans H, Van den Bogaert W, Barillot I, et al. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med. 2001; 345:1378–87.
Article2. Bartelink H, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007; 25:3259–65.
Article3. Kirova YM, Fournier-Bidoz N, Servois V, Laki F, Pollet GA, Salmon R, et al. How to boost the breast tumor bed? A multidisciplinary approach in eight steps. Int J Radiat Oncol Biol Phys. 2008; 72:494–500.
Article4. Lee R, Chung E, Lee J, Suh H. Evaluation of electron boost fields based on surgical clips and operative scars in definitive breast irradiation. J Korean Soc Ther Radiol Oncol. 2005; 23:236–42.5. Goldberg H, Prosnitz RG, Olson JA, Marks LB. Definition of postlumpectomy tumor bed for radiotherapy boost field planning: CT versus surgical clips. Int J Radiat Oncol Biol Phys. 2005; 63:209–13.
Article6. Bedwinek J. Breast conserving surgery and irradiation: the importance of demarcating the excision cavity with surgical clips. Int J Radiat Oncol Biol Phys. 1993; 26:675–9.
Article7. Hunter MA, McFall TA, Hehr KA. Breast-conserving surgery for primary breast cancer: necessity for surgical clips to define the tumor bed for radiation planning. Radiology. 1996; 200:281–2.
Article8. Harrington KJ, Harrison M, Bayle P, Evans K, Dunn PA, Lambert HE, et al. Surgical clips in planning the electron boost in breast cancer: a qualitative and quantitative evaluation. Int J Radiat Oncol Biol Phys. 1996; 34:579–84.
Article9. Sung S, Lee JH, Lee JH, Kim SH, Kwak YK, Lee SW, et al. Displacement of surgical clips during postoperative radiotherapy in breast cancer patients who received breast-conserving surgery. J Breast Cancer. 2016; 19:417–22.
Article10. Hepel JT, Evans SB, Hiatt JR, Price LL, DiPetrillo T, Wazer DE, et al. Planning the breast boost: comparison of three techniques and evolution of tumor bed during treatment. Int J Radiat Oncol Biol Phys. 2009; 74:458–63.
Article11. Harris EJ, Donovan EM, Yarnold JR, Coles CE, Evans PM; IMPORT Trial Management Group. Characterization of target volume changes during breast radiotherapy using implanted fiducial markers and portal imaging. Int J Radiat Oncol Biol Phys. 2009; 73:958–66.
Article12. Oh KS, Kong FM, Griffith KA, Yanke B, Pierce LJ. Planning the breast tumor bed boost: changes in the excision cavity volume and surgical scar location after breast-conserving surgery and whole-breast irradiation. Int J Radiat Oncol Biol Phys. 2006; 66:680–6.
Article13. Inaji H, Yanagisawa T, Komoike Y, Motomura K, Koyama H. Wide excision as a method of breast-conserving surgery for breast cancer. Nihon Geka Gakkai Zasshi. 2002; 103:806–10.14. Leong C, Boyages J, Jayasinghe UW, Bilous M, Ung O, Chua B, et al. Effect of margins on ipsilateral breast tumor recurrence after breast conservation therapy for lymph node-negative breast carcinoma. Cancer. 2004; 100:1823–32.
Article15. Horiguchi J, Iino Y, Takei H, Maemura M, Yokoe T, Niibe H, et al. Surgical margin and breast recurrence after breast-conserving therapy. Oncol Rep. 1999; 6:135–8.
Article16. Wazer DE, DiPetrillo T, Schmidt-Ullrich R, Weld L, Smith TJ, Marchant DJ, et al. Factors influencing cosmetic outcome and complication risk after conservative surgery and radiotherapy for early-stage breast carcinoma. J Clin Oncol. 1992; 10:356–63.
Article17. Denham JW, Sillar RW, Clarke D. Boost dosage to the excision site following conservative surgery for breast cancer: it's easy to miss! Clin Oncol (R Coll Radiol). 1991; 3:257–61.
Article18. Prendergast B, Indelicato DJ, Grobmyer SR, Saito AI, Lightsey JL, Snead FE, et al. The dynamic tumor bed: volumetric changes in the lumpectomy cavity during breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2009; 74:695–701.
Article19. Chung MJ, Suh YJ, Lee HC, Kang DG, Kim EJ, Kim SH, et al. Tumor bed volumetric changes during breast irradiation for the patients with breast cancer. Radiat Oncol J. 2013; 31:228–33.
Article20. Hernanz F, Regano S, Redondo-Figuero C, Orallo V, Erasun F, Gomez-Fleitas M. Oncoplastic breast-conserving surgery: analysis of quadrantectomy and immediate reconstruction with latissimus dorsi flap. World J Surg. 2007; 31:1934–40.
Article21. Alco G, Igdem S, Okkan S, Dincer M, Sarsenov D, Ilgun AS, et al. Replacement of the tumor bed following oncoplastic breast-conserving surgery with immediate latissimus dorsi mini-flap. Mol Clin Oncol. 2016; 5:365–71.22. Jordan SW, Khavanin N, Kim JY. Seroma in prosthetic breast reconstruction. Plast Reconstr Surg. 2016; 137:1104–16.
Article23. Weed DW, Yan D, Martinez AA, Vicini FA, Wilkinson TJ, Wong J. The validity of surgical clips as a radiographic surrogate for the lumpectomy cavity in image-guided accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2004; 60:484–92.
Article24. Petersen RP, Truong PT, Kader HA, Berthelet E, Lee JC, Hilts ML, et al. Target volume delineation for partial breast radiotherapy planning: clinical characteristics associated with low interobserver concordance. Int J Radiat Oncol Biol Phys. 2007; 69:41–8.
Article25. Sager O, Dincoglan F, Uysal B, Demiral S, Gamsiz H, Elcim Y, et al. Evaluation of adaptive radiotherapy (ART) by use of replanning the tumor bed boost with repeated computed tomography (CT) simulation after whole breast irradiation (WBI) for breast cancer patients having clinically evident seroma. Jpn J Radiol. 2018; 36:401–6.
Article26. Alderliesten T, den Hollander S, Yang TJ, Elkhuizen PH, van Mourik AM, Hurkmans C, et al. Dosimetric impact of postoperative seroma reduction during radiotherapy after breast-conserving surgery. Radiother Oncol. 2011; 100:265–70.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Displacement of Surgical Clips during Postoperative Radiotherapy in Breast Cancer Patients Who Received Breast-Conserving Surgery
- Follow-Up after Volume Replacement Using Acellular Dermal Matrix in Oncoplastic Breast-Conserving Surgery
- Comparison of Psychiatric Symptoms between Total Mastectomy and Breast Conserving Surgery in Breast Cancer Patients
- Comparison of Wound Closure Using Acellular Dermal Matrix With Primary Wound Closure After Breast Conserving Surgery in Breast Cancer Patients
- Volumetric changes in the lumpectomy cavity during whole breast irradiation after breast conserving surgery