Clin Exp Otorhinolaryngol.
2020 May;13(2):106-112. 10.21053/ceo.2019.00766.
Extratympanic Observation of Middle and Inner Ear Structures in Rodents Using Optical Coherence Tomography
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Pusan National University Hospital, Pusan National University School of Medicine and Medical Research Institute, Busan, Korea
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea
- 3Koh Young Technology Inc., Seoul, Korea
- KMID: 2500282
- DOI: http://doi.org/10.21053/ceo.2019.00766
Abstract
Objectives
. This study aimed to investigate whether optical coherence tomography (OCT) provides useful information about the microstructures of the middle and inner ear via extratympanic approach and thereby could be utilized as an alternative diagnostic technology in ear imaging.
Methods
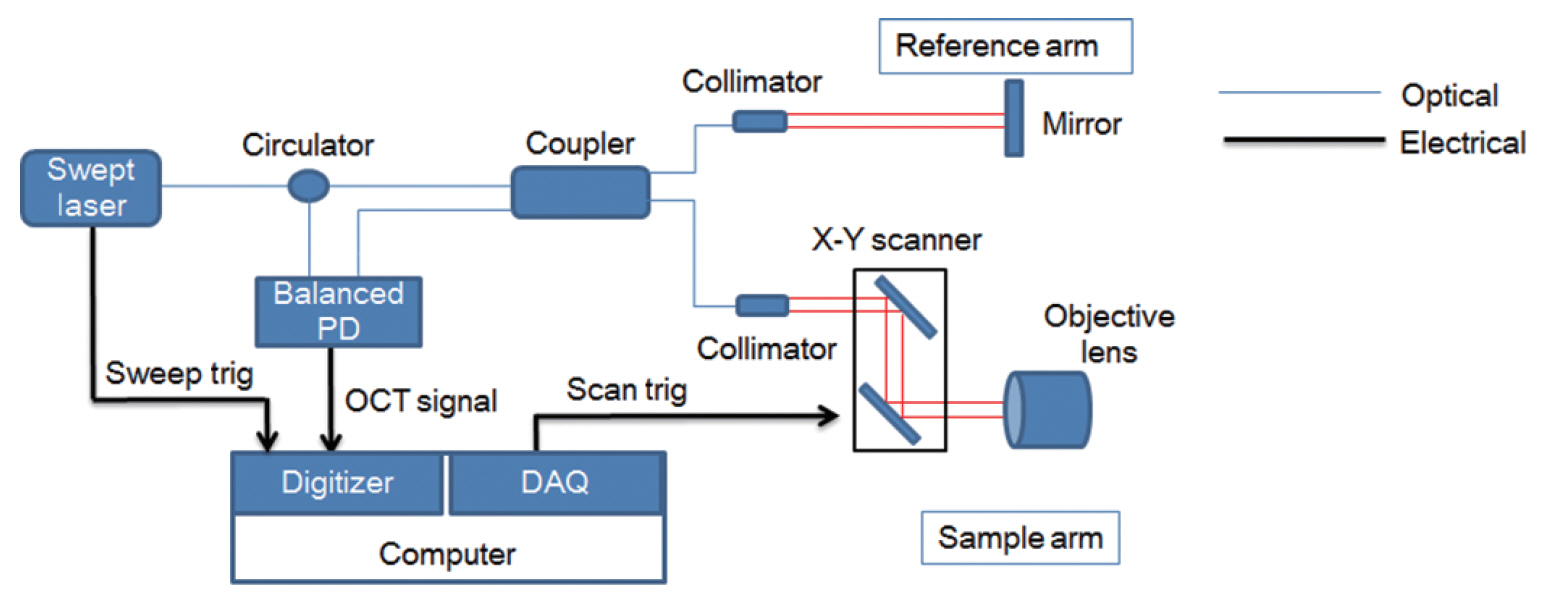
. Five rats and mice were included, and the swept-source OCT system was applied to confirm the extent of visibility of the middle and inner ear and measure the length or thickness of the microstructures in the ear. The cochlea was subsequently dissected following OCT and histologically evaluated to compare with the OCT images.
Results
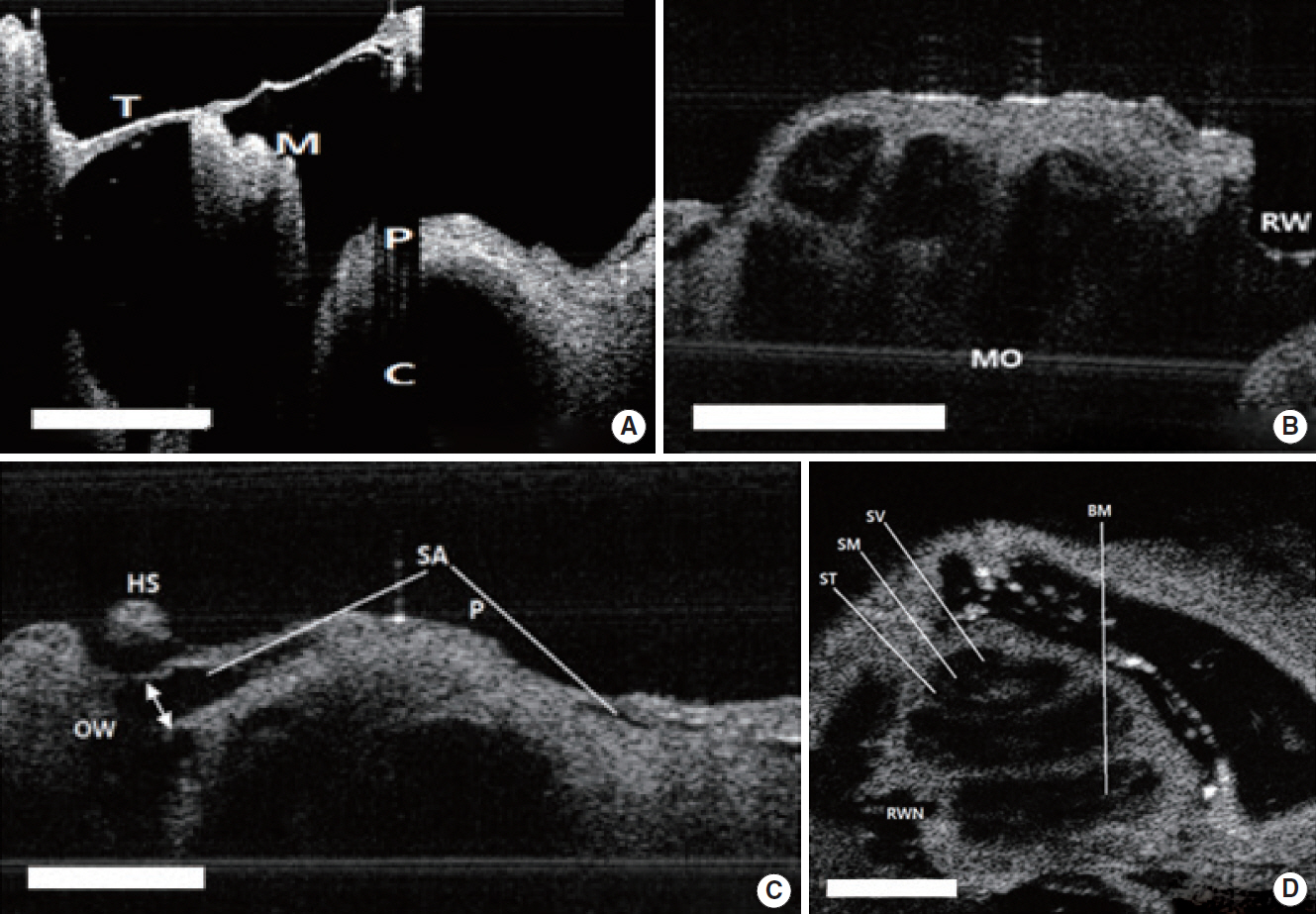
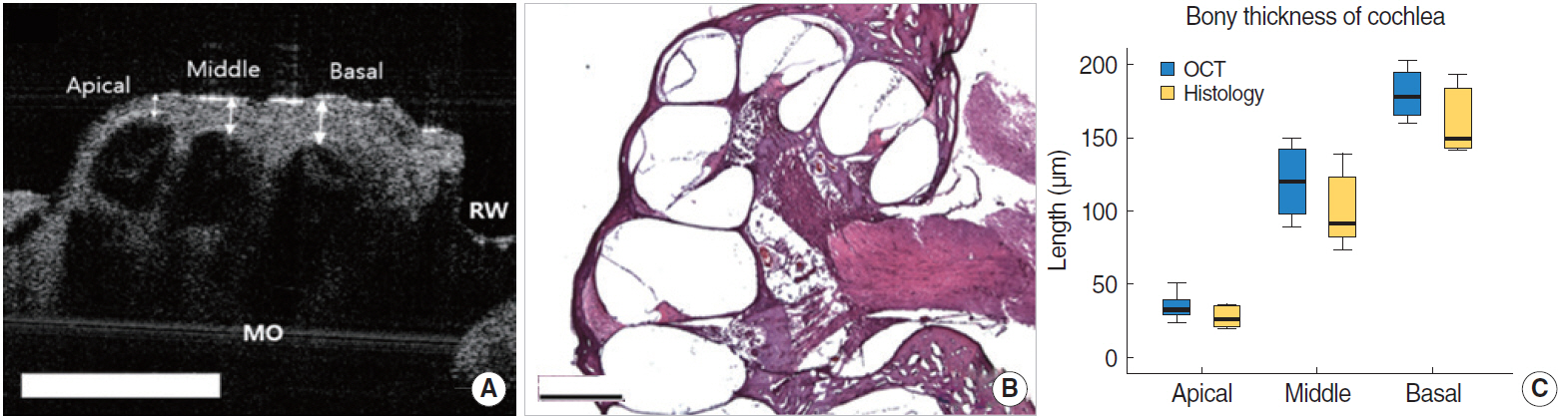
. The middle ear microstructures such as ossicles, stapedial artery and oval window through the tympanic membrane with the OCT could be confirmed in both rats and mice. It was also possible to obtain the inner ear images such as each compartment of the cochlea in the mice, but the bone covering bulla needed to be removed to visualize the inner ear structures in the rats which had thicker bulla. The bony thickness covering the cochlea could be measured, which showed no significant differences between OCT and histologic image at all turns of cochlea.
Conclusion
. OCT has been shown a promising technology to assess real-time middle and inner ear microstructures noninvasively with a high-resolution in the animal model. Therefore, OCT could be utilized to provide additional diagnostic information about the diseases of the middle and inner ear.
Figure
Cited by 1 articles
-
Future Directions of Optical Coherence Tomography in Otology: A Morphological and Functional Approach
Nam Hyun Cho, Jeong Hun Jang
Clin Exp Otorhinolaryngol. 2020;13(2):85-86. doi: 10.21053/ceo.2020.00031.
Reference
-
1. Monroy GL, Shelton RL, Nolan RM, Nguyen CT, Novak MA, Hill MC, et al. Noninvasive depth-resolved optical measurements of the tympanic membrane and middle ear for differentiating otitis media. Laryngoscope. 2015; Aug. 125(8):E276–82.
Article2. Rainsbury JW, Landry TG, Brown JA, Adamson RA, Bance M. High frequency ex vivo ultrasound imaging of the middle ear to show simulated ossicular pathology. Otol Neurotol. 2016; Jun. 37(5):586–92.
Article3. Sennaroglu L, Saatci I, Aralasmak A, Gursel B, Turan E. Magnetic resonance imaging versus computed tomography in pre-operative evaluation of cochlear implant candidates with congenital hearing loss. J Laryngol Otol. 2002; Oct. 116(10):804–10.
Article4. Fujimoto JG, Farkas D. Biomedical optical imaging. Oxford, UK: Oxford University Press;2009.
Article5. Cho NH, Jang JH, Jung W, Kim J. In vivo imaging of middle-ear and inner-ear microstructures of a mouse guided by SD-OCT combined with a surgical microscope. Opt Express. 2014; Apr. 22(8):8985–95.6. Just T, Lankenau E, Hüttmann G, Pau HW. Optical coherence tomography of the oval window niche. J Laryngol Otol. 2009; Jun. 123(6):603–8.
Article7. Chang EW, Cheng JT, Roosli C, Kobler JB, Rosowski JJ, Yun SH. Simultaneous 3D imaging of sound-induced motions of the tympanic membrane and middle ear ossicles. Hear Res. 2013; Oct. 304:49–56.
Article8. Gao SS, Raphael PD, Wang R, Park J, Xia A, Applegate BE, et al. In vivo vibrometry inside the apex of the mouse cochlea using spectral domain optical coherence tomography. Biomed Opt Express. 2013; Feb. 4(2):230–40.9. Subhash HM, Choudhury N, Chen F, Wang RK, Jacques SL, Nuttall AL. Depth-resolved dual-beamlet vibrometry based on Fourier domain low coherence interferometry. J Biomed Opt. 2013; Mar. 18(3):036003.
Article10. Tan HE, Santa Maria PL, Wijesinghe P, Francis Kennedy B, Allardyce BJ, Eikelboom RH, et al. Optical coherence tomography of the tympanic membrane and middle ear: a review. Otolaryngol Head Neck Surg. 2018; Sep. 159(3):424–38.
Article11. Jungheim M, Donner S, Bleeker S, Ripken T, Krueger A, Ptok M. Effect of saline inhalation on vocal fold epithelial morphology evaluated by optical coherence tomography. Laryngoscope. 2016; Oct. 126(10):E332–6.
Article12. Pitris C, Saunders KT, Fujimoto JG, Brezinski ME. High-resolution imaging of the middle ear with optical coherence tomography: a feasibility study. Arch Otolaryngol Head Neck Surg. 2001; Jun. 127(6):637–42.13. Ruah CB, Schachern PA, Zelterman D, Paparella MM, Yoon TH. Age-related morphologic changes in the human tympanic membrane: a light and electron microscopic study. Arch Otolaryngol Head Neck Surg. 1991; Jun. 117(6):627–34.
Article14. Djalilian HR, Ridgway J, Tam M, Sepehr A, Chen Z, Wong BJ. Imaging the human tympanic membrane using optical coherence tomography in vivo. Otol Neurotol. 2008; Dec. 29(8):1091–4.
Article15. MacDougall D, Rainsbury J, Brown J, Bance M, Adamson R. Optical coherence tomography system requirements for clinical diagnostic middle ear imaging. J Biomed Opt. 2015; May. 20(5):56008.
Article16. Tona Y, Sakamoto T, Nakagawa T, Adachi T, Taniguchi M, Torii H, et al. In vivo imaging of mouse cochlea by optical coherence tomography. Otol Neurotol. 2014; Feb. 35(2):e84–9.17. Pawlowski ME, Shrestha S, Park J, Applegate BE, Oghalai JS, Tkaczyk TS. Miniature, minimally invasive, tunable endoscope for investigation of the middle ear. Biomed Opt Express. 2015; May. 6(6):2246–57.
Article18. Lee J, Wijesinghe RE, Jeon D, Kim P, Choung YH, Jang JH, et al. Clinical utility of intraoperative tympanomastoidectomy assessment using a surgical microscope integrated with an optical coherence tomography. Sci Rep. 2018; Nov. 8(1):17432.
Article19. Kirsten L, Schindler M, Morgenstern J, Erkkila MT, Golde J, Walther J, et al. Endoscopic optical coherence tomography with wide field-of-view for the morphological and functional assessment of the human tympanic membrane. J Biomed Opt. 2018; Dec. 24(3):1–11.
Article20. Tao YK, Srivastava SK, Ehlers JP. Microscope-integrated intraoperative OCT with electrically tunable focus and heads-up display for imaging of ophthalmic surgical maneuvers. Biomed Opt Express. 2014; May. 5(6):1877–85.
Article21. Garcia JA, Benboujja F, Beaudette K, Guo R, Boudoux C, Hartnick CJ. Using attenuation coefficients from optical coherence tomography as markers of vocal fold maturation. Laryngoscope. 2016; Jun. 126(6):E218–23.
Article22. Drexler W, Liu M, Kumar A, Kamali T, Unterhuber A, Leitgeb RA. Optical coherence tomography today: speed, contrast, and multimodality. J Biomed Opt. 2014; 19(7):071412.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Availability of Optical Coherence Tomography in Diagnosis and Classification of Choroidal Neovascularization
- Optimization of Percutaneous Coronary Intervention Using Optical Coherence Tomography
- Optical Coherence Tomography in the Evalution of Fitering Bleb after Trabeculectomy
- Optical Imaging and Its Clinical Application in Otorhinolaryngology
- Measurement of Deep Optic Nerve Complex Structures with Two Spectral Domain Optical Coherence Tomography Instruments