J Korean Med Sci.
2020 Apr;35(15):e106. 10.3346/jkms.2020.35.e106.
Iatrogenic Opioid Withdrawal Syndrome in Critically Ill Patients: a Retrospective Cohort Study
- Affiliations
-
- 1Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2500191
- DOI: http://doi.org/10.3346/jkms.2020.35.e106
Abstract
- Background
Opioid withdrawal syndrome (OWS) may occur following the reduction or discontinuation of opioid analgesics. In critically ill pediatric patients, OWS is a common and clinically significant condition. However, OWS in adult patients has not been assessed in detail. Therefore, we aimed to investigate the incidence, risk factors, and clinical features of OWS in mechanically ventilated patients treated in an adult intensive care unit (ICU).
Methods
This study was a retrospective evaluation of data from patients treated in the medical ICU for > 3 days and who received only one type of opioid analgesic. OWS was assessed over a 24 hours period from discontinuation or reduction (by > 50%) of continuous opioid infusion. OWS was defined as the presence of ≥ 3 central nervous system or autonomic nervous system symptoms.
Results
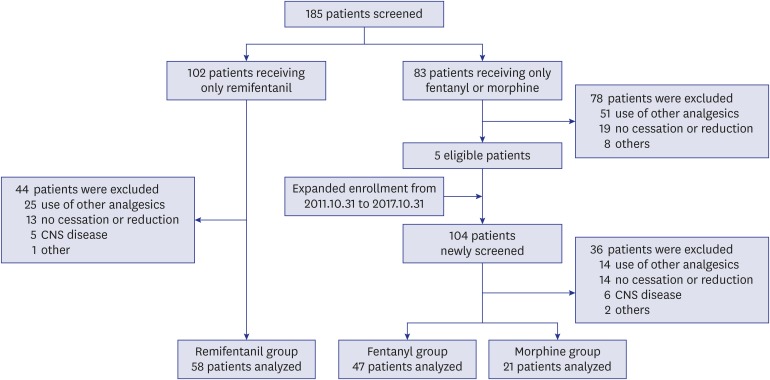
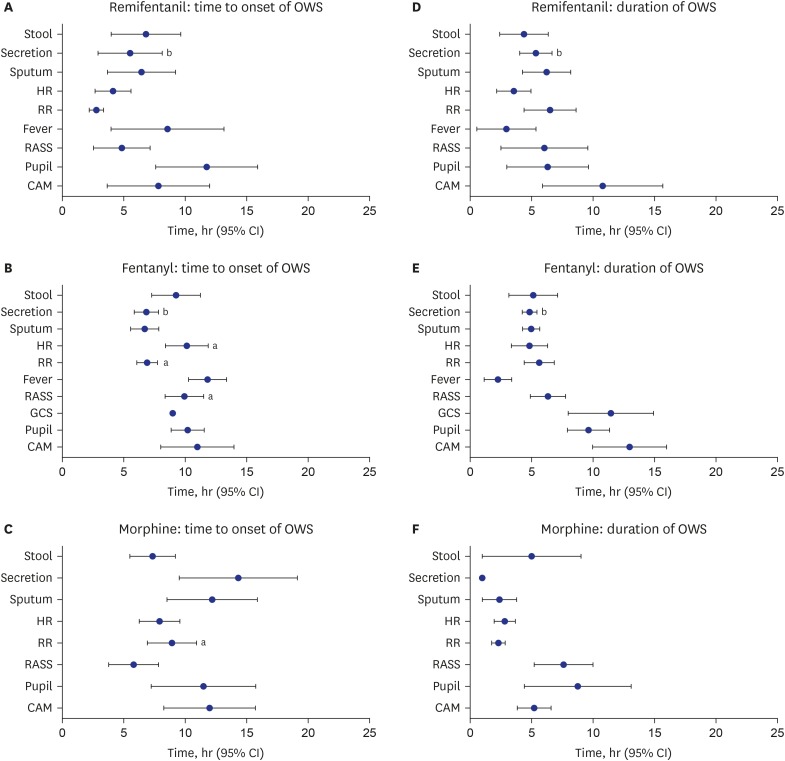
In 126 patients treated with remifentanil (n = 58), fentanyl (n = 47), or morphine (n = 21), OWS was seen in 31.0%, 36.2%, and 9.5% of patients, respectively (P = 0.078). The most common symptom was a change in respiratory rate (remifentanil, 94.4%; fentanyl, 76.5%; morphine, 100%). Multivariate Cox-proportional hazards model showed that OWS was negatively associated with morphine treatment (hazard ratio [HR], 0.17; 95% confidence interval [CI], 0.037–0.743) and duration of opioid infusion (HR, 0.566; 95% CI, 0.451–0.712).
Conclusion
OWS is not uncommon in mechanically ventilated adult patients who received continuous infusion of opioids for > 3 days. The use of morphine may be associated with a decreased risk of OWS.
Figure
Reference
-
1. Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013; 41(1):263–306. PMID: 23269131.
Article2. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002; 1(1):13–20. PMID: 18567959.
Article3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. Arlington, VA: American Psychiatric Publishing;2013.4. Amigoni A, Mondardini MC, Vittadello I, Zaglia F, Rossetti E, Vitale F, et al. Withdrawal assessment tool-1 monitoring in PICU: a multicenter study on iatrogenic withdrawal syndrome. Pediatr Crit Care Med. 2017; 18(2):e86–91. PMID: 28157809.5. Maguire D, Cline GJ, Parnell L, Tai CY. Validation of the Finnegan neonatal abstinence syndrome tool-short form. Adv Neonatal Care. 2013; 13(6):430–437. PMID: 24300963.
Article6. Birchley G. Opioid and benzodiazepine withdrawal syndromes in the paediatric intensive care unit: a review of recent literature. Nurs Crit Care. 2009; 14(1):26–37. PMID: 19154308.
Article7. Katz R, Kelly HW, Hsi A. Prospective study on the occurrence of withdrawal in critically ill children who receive fentanyl by continuous infusion. Crit Care Med. 1994; 22(5):763–767. PMID: 8181283.
Article8. Anand KJ, Willson DF, Berger J, Harrison R, Meert KL, Zimmerman J, et al. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics. 2010; 125(5):e1208–25. PMID: 20403936.
Article9. Fonsmark L, Rasmussen YH, Carl P. Occurrence of withdrawal in critically ill sedated children. Crit Care Med. 1999; 27(1):196–199. PMID: 9934916.
Article10. Ducharme C, Carnevale FA, Clermont MS, Shea S. A prospective study of adverse reactions to the weaning of opioids and benzodiazepines among critically ill children. Intensive Crit Care Nurs. 2005; 21(3):179–186. PMID: 15907670.
Article11. Best KM, Boullata JI, Curley MA. Risk factors associated with iatrogenic opioid and benzodiazepine withdrawal in critically ill pediatric patients: a systematic review and conceptual model. Pediatr Crit Care Med. 2015; 16(2):175–183. PMID: 25560429.12. Cammarano WB, Pittet JF, Weitz S, Schlobohm RM, Marks JD. Acute withdrawal syndrome related to the administration of analgesic and sedative medications in adult intensive care unit patients. Crit Care Med. 1998; 26(4):676–684. PMID: 9559604.
Article13. Wang PP, Huang E, Feng X, Bray CA, Perreault MM, Rico P, et al. Opioid-associated iatrogenic withdrawal in critically ill adult patients: a multicenter prospective observational study. Ann Intensive Care. 2017; 7(1):88. PMID: 28866754.
Article14. Karabinis A, Mandragos K, Stergiopoulos S, Komnos A, Soukup J, Speelberg B, et al. Safety and efficacy of analgesia-based sedation with remifentanil versus standard hypnotic-based regimens in intensive care unit patients with brain injuries: a randomised, controlled trial [ISRCTN50308308]. Crit Care. 2004; 8(4):R268–80. PMID: 15312228.15. Tan JA, Ho KM. Use of remifentanil as a sedative agent in critically ill adult patients: a meta-analysis. Anaesthesia. 2009; 64(12):1342–1352. PMID: 19849681.
Article16. Best KM, Wypij D, Asaro LA, Curley MA. Randomized Evaluation of Sedation Titration For Respiratory Failure Study Investigators. Patient, process, and system predictors of iatrogenic withdrawal syndrome in critically ill children. Crit Care Med. 2017; 45(1):e7–15. PMID: 27513532.
Article17. Ista E, de Hoog M, Tibboel D, Duivenvoorden HJ, van Dijk M. Psychometric evaluation of the Sophia observation withdrawal symptoms scale in critically ill children. Pediatr Crit Care Med. 2013; 14(8):761–769. PMID: 23962832.
Article18. Hughes MA, Glass PS, Jacobs JR. Context-sensitive half-time in multicompartment pharmacokinetic models for intravenous anesthetic drugs. Anesthesiology. 1992; 76(3):334–341. PMID: 1539843.19. Egan TD, Minto CF, Hermann DJ, Barr J, Muir KT, Shafer SL. Remifentanil versus alfentanil: comparative pharmacokinetics and pharmacodynamics in healthy adult male volunteers. Anesthesiology. 1996; 84(4):821–833. PMID: 8638836.21. Dominguez KD, Lomako DM, Katz RW, Kelly HW. Opioid withdrawal in critically ill neonates. Ann Pharmacother. 2003; 37(4):473–477. PMID: 12659598.
Article22. Arnold JH, Truog RD, Orav EJ, Scavone JM, Hershenson MB. Tolerance and dependence in neonates sedated with fentanyl during extracorporeal membrane oxygenation. Anesthesiology. 1990; 73(6):1136–1140. PMID: 2248393.
Article23. Gupta A, Singh-Radcliff N. Pharmacology in Anesthesia Practice. New York: Oxford University Press;2013.24. Delvaux B, Ryckwaert Y, Van Boven M, De Kock M, Capdevila X. Remifentanil in the intensive care unit: tolerance and acute withdrawal syndrome after prolonged sedation. Anesthesiology. 2005; 102(6):1281–1282. PMID: 15915043.
Article25. Franck LS, Vilardi J, Durand D, Powers R. Opioid withdrawal in neonates after continuous infusions of morphine or fentanyl during extracorporeal membrane oxygenation. Am J Crit Care. 1998; 7(5):364–369. PMID: 9740886.
Article26. Tobias JD. Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Crit Care Med. 2000; 28(6):2122–2132. PMID: 10890677.
Article27. Bot G, Blake AD, Li S, Reisine T. Fentanyl and its analogs desensitize the cloned mu opioid receptor. J Pharmacol Exp Ther. 1998; 285(3):1207–1218. PMID: 9618424.28. Noble F, Cox BM. Differential desensitization of mu- and delta- opioid receptors in selected neural pathways following chronic morphine treatment. Br J Pharmacol. 1996; 117(1):161–169. PMID: 8825358.29. Blake AD, Bot G, Freeman JC, Reisine T. Differential opioid agonist regulation of the mouse mu opioid receptor. J Biol Chem. 1997; 272(2):782–790. PMID: 8995364.30. Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJ, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018; 46(9):e825–73. PMID: 30113379.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Withdrawal Symptom after Injection of Nalbuphine in a Chronically Morphine-Dependent Patient
- Impact of Interhospital Transfer in Mortality of Critically Ill Patients
- Opioid Withdrawal Symptoms after Conversion to Oral Oxycodone/Naloxone in Advanced Cancer Patients Receiving Strong Opioids
- Changes of the level of G protein alpha-subunit mRNA by withdrawal from morphine and butorphanol
- A Case of Pancreatic Cancer and Opioid Withdrawal after Endoscopic Ultrasound-guided Celiac Plexus Neurolysis