Endocrinol Metab.
2020 Mar;35(1):142-148. 10.3803/EnM.2020.35.1.142.
Comparison of the Efficacy and Safety of Insulin Detemir Administered Once Daily According to Two Titration Algorithms (3-0-3 and 2-4-6-8) in Patients with Type 2 Diabetes Mellitus
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Daejeon Eulji Medical Center, Eulji University School of Medicine, Daejeon, Korea. pkkss@eulji.ac.kr
- 2Department of Endocrinology and Metabolism, Konyang University Hospital, Konyang University College of Medicine, Daejeon, Korea.
- 3Department of Endocrinology and Metabolism, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Daejeon, Korea.
- 4Department of Endocrinology and Metabolism, Chungnam National University Hospital, Chungnam National University College of Medicine, Daejeon, Korea.
- 5Department of Endocrinology and Metabolism, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 6Department of Endocrinology and Metabolism, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea.
- KMID: 2471730
- DOI: http://doi.org/10.3803/EnM.2020.35.1.142
Abstract
- BACKGROUND
This study was conducted to compare glycaemic control with insulin detemir administered according to two titration algorithms (3-0-3 and 2-4-6-8) after 20 weeks of treatment in subjects with type 2 diabetes mellitus inadequately controlled on metformin.
METHODS
This was a 20-week, randomised, multicentre, open-labelled, treat-to-target trial. Forty-six patients were randomised in a 1:1 manner to either the 3-0-3 (G3, n=23) or 2-4-6-8 (G2, n=23) algorithm. The primary endpoint was change of haemoglobin A1c (HbA1c), and the secondary safety endpoint included hypoglycaemic events.
RESULTS
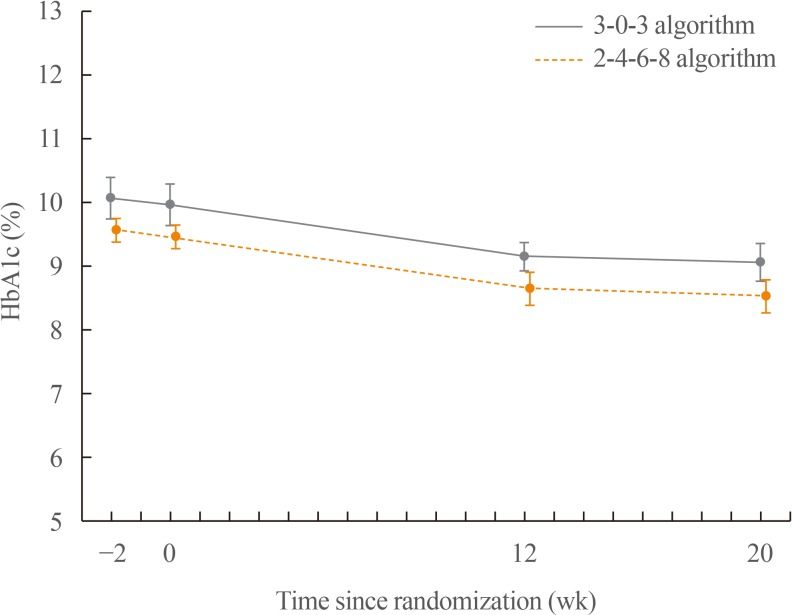
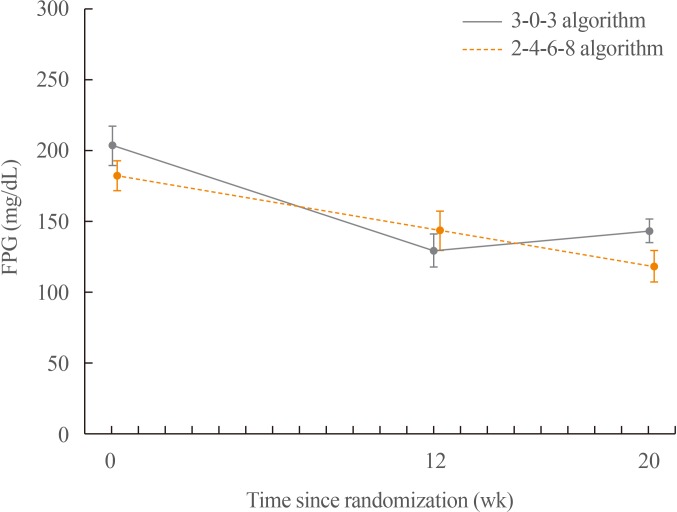
After 20 weeks, HbA1c decreased similarly in the G3 and G2 groups, with a mean change of −0.9% from baseline. The mean change in fasting plasma glucose was numerically similar in both groups. The hypoglycaemia event rate per 100-patient-years of exposure (r) in the G2 group (r=1,427) was higher than that in the G3 group (r=807).
CONCLUSION
Both treatment groups had numerically similar HbA1c reductions. A trend towards fewer hypoglycaemia episodes after dose stabilisation was seen with the simpler G3. Clinically, this may be an important observation, as a simpler titration algorithm may support self-management and maintenance of insulin therapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Home P, Naggar NE, Khamseh M, Gonzalez-Galvez G, Shen C, Chakkarwar P, et al. An observational non-interventional study of people with diabetes beginning or changed to insulin analogue therapy in non-Western countries: the A1chieve study. Diabetes Res Clin Pract. 2011; 94:352–363. PMID: 22153567.
Article2. Riddle MC, Rosenstock J, Gerich J. Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003; 26:3080–3086. PMID: 14578243.3. Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006; 28:1569–1581. PMID: 17157113.
Article4. Jabbour S. Primary care physicians and insulin initiation: multiple barriers, lack of knowledge or both? Int J Clin Pract. 2008; 62:845–847. PMID: 18479275.
Article5. Fajardo Montanana C, Hernandez Herrero C, Rivas Fernandez M. Less weight gain and hypoglycaemia with once-daily insulin detemir than NPH insulin in intensification of insulin therapy in overweight type 2 diabetes patients: the PREDICTIVE BMI clinical trial. Diabet Med. 2008; 25:916–923. PMID: 18959604.6. Caputo S, Andersen H, Kaiser M, Karnieli E, Meneghini LF, Svendsen AL, et al. Effect of baseline glycosylated hemoglobin A1c on glycemic control and diabetes management following initiation of once-daily insulin detemir in real-life clinical practice. Endocr Pract. 2013; 19:462–470. PMID: 23337147.
Article7. Hollander P, Raslova K, Skjoth TV, Rastam J, Liutkus JF. Efficacy and safety of insulin detemir once daily in combination with sitagliptin and metformin: the TRANSITION randomized controlled trial. Diabetes Obes Metab. 2011; 13:268–275. PMID: 21205123.
Article8. Le Floch JP, Levy M, Mosnier-Pudar H, Nobels F, Laroche S, Gonbert S, et al. Comparison of once-versus twice-daily administration of insulin detemir, used with mealtime insulin aspart, in basal-bolus therapy for type 1 diabetes: assessment of detemir administration in a progressive treat-to-target trial (ADAPT). Diabetes Care. 2009; 32:32–37. PMID: 18945928.9. Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R. ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005; 28:1282–1288. PMID: 15920040.10. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008; 359:1577–1589. PMID: 18784090.
Article11. Blonde L, Merilainen M, Karwe V, Raskin P. TITRATE Study Group. Patient-directed titration for achieving glycaemic goals using a once-daily basal insulin analogue: an assessment of two different fasting plasma glucose targets. The TITRATE study. Diabetes Obes Metab. 2009; 11:623–631. PMID: 19515182.12. Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993; 329:977–986. PMID: 8366922.
Article13. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998; 352:837–853. PMID: 9742976.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of insulin detemir in dogs with diabetes mellitus
- Efficacy and Safety of Self-Titration Algorithms of Insulin Glargine 300 units/mL in Individuals with Uncontrolled Type 2 Diabetes Mellitus (The Korean TITRATION Study): A Randomized Controlled Trial
- Pharmacothearpy of Adolescents with Diabetes
- Summary of Insulin Therapy for Adult Patients with Type 2 Diabetes Mellitus: A Position Statement of the Korean Diabetes Association, 2017
- Nutritional Management and Carbohydrate Counting for Patients with Type 1 Diabetes Mellitus