Ann Hepatobiliary Pancreat Surg.
2020 Feb;24(1):97-103. 10.14701/ahbps.2020.24.1.97.
Robotic enucleation of a pancreatic uncinate neuroendocrine tumor – a unique parenchyma-saving strategy for uncinate tumors
- Affiliations
-
- 1Department of Hepatopancreatobiliary and Transplant Surgery, Singapore General Hospital, Singapore. bsgkp@hotmail.com
- 2Duke-National University of Singapore (NUS) Medical School, Singapore.
- KMID: 2471197
- DOI: http://doi.org/10.14701/ahbps.2020.24.1.97
Abstract
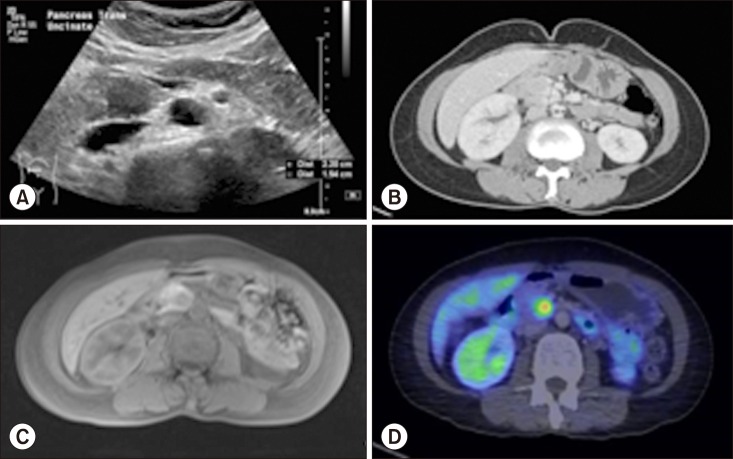
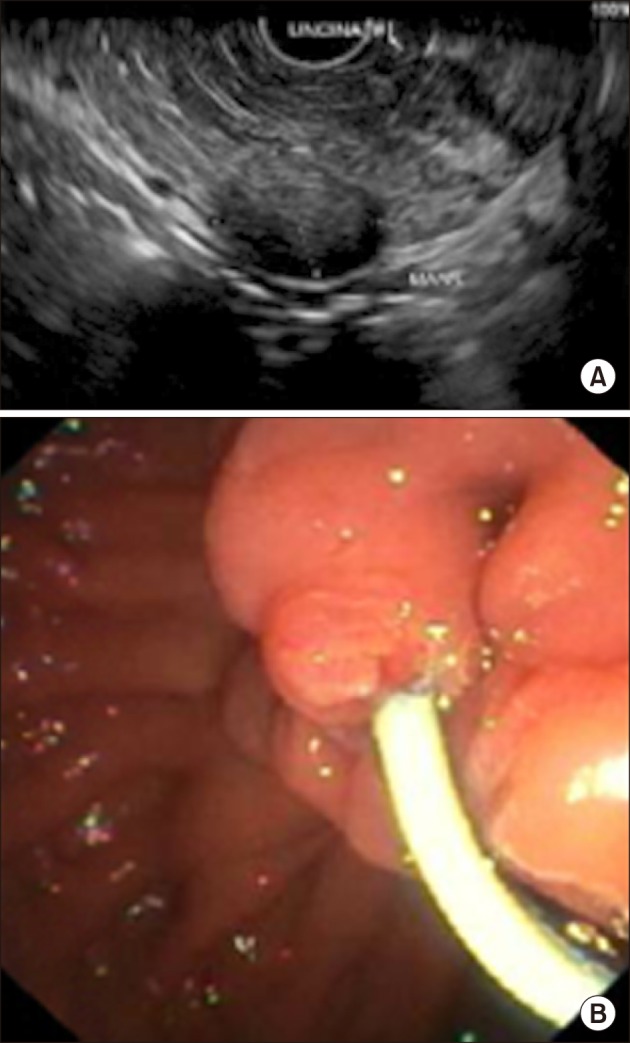
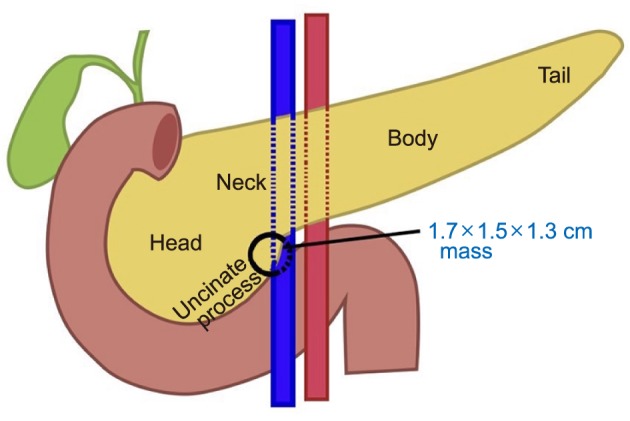
- Pancreatic neuroendocrine tumors (PNET) comprise up to 10% of all pancreatic solid tumors. There has been much interest in recent years with regards to the role of limited resection and enucleation procedures for this entity. There is no clear guideline today on the optimal type choice of surgery for this condition, with even fewer reporting on the use of a robotic approach for pancreatic uncinate lesions. We describe a case report of a 54-year-old lady who underwent successful robotic enucleation of pancreatic uncinate neuroendocrine tumor. This patient's recovery was complicated by pancreatitis and a peripancreatic collection, both of which resolved without surgical re-intervention. A literature review was performed with regards to current guidelines on management of PNETs, comparisons between demolitive and parenchymal-preserving procedures, and recent developments in the laparoscopic and robotic approaches for this condition. There is no clear guideline on the optimal type and approach (open vs. laparoscopic vs. robotic) to the surgical management of PNET. We document in this case report a novel approach of robotic enucleation of pancreatic uncinate process NET, that could be considered as an alternative to open/laparoscopic demolitive procedures for small uncinate tumors.
Keyword
MeSH Terms
Figure
Reference
-
1. Bartolini I, Bencini L, Risaliti M, Ringressi MN, Moraldi L, Taddei A. Current management of pancreatic neuroendocrine tumors: from demolitive surgery to observation. Gastroenterol Res Pract. 2018; 2018:9647247. PMID: 30140282.
Article2. Cheema A, Weber J, Strosberg JR. Incidental detection of pancreatic neuroendocrine tumors: an analysis of incidence and outcomes. Ann Surg Oncol. 2012; 19:2932–2936. PMID: 22350605.
Article3. Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, et al. ; Vienna Consensus Conference participants. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016; 103:153–171. PMID: 26742109.4. Lloyd RV, Osamura RY, Klöppel G, Rosai J, Bosman FT, Jaffe ES, et al. WHO classification of tumours of endocrine organs. 4th ed. Lyon: International Agency for Research on Cancer;2017.5. Bilimoria KY, Talamonti MS, Tomlinson JS, Stewart AK, Winchester DP, Ko CY, et al. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg. 2008; 247:490–500. PMID: 18376195.6. Teo RYA, Teo TZ, Tai DWM, Tan DM, Ong S, Goh BKP. Systematic review of current prognostication systems for pancreatic neuroendocrine neoplasms. Surgery. 2019; 165:672–685. PMID: 30558808.
Article7. Shah MH, Goldner WS, Halfdanarson TR, Bergsland E, Berlin JD, Halperin D, et al. NCCN guidelines insights: neuroendocrine and adrenal tumors, version 2.2018. J Natl Compr Canc Netw. 2018; 16:693–702. PMID: 29891520.
Article8. Singh S, Dey C, Kennecke H, Kocha W, Maroun J, Metrakos P, et al. Consensus recommendations for the diagnosis and management of pancreatic neuroendocrine tumors: guidelines from a Canadian National Expert Group. Ann Surg Oncol. 2015; 22:2685–2699. PMID: 25366583.
Article9. Jilesen AP, van Eijck CH, Busch OR, van Gulik TM, Gouma DJ, van Dijkum EJ. Postoperative outcomes of enucleation and standard resections in patients with a pancreatic neuroendocrine tumor. World J Surg. 2016; 40:715–728. PMID: 26608956.
Article10. Falconi M, Zerbi A, Crippa S, Balzano G, Boninsegna L, Capitanio V, et al. Parenchyma-preserving resections for small nonfunctioning pancreatic endocrine tumors. Ann Surg Oncol. 2010; 17:1621–1627. PMID: 20162460.
Article11. Zhou Y, Zhao M, Wu L, Ye F, Si X. Short- and long-term outcomes after enucleation of pancreatic tumors: an evidence-based assessment. Pancreatology. 2016; 16:1092–1098. PMID: 27423534.
Article12. Teo RYA, Goh BKP. Surgical resection of pancreatic neuroendocrine neoplasm by minimally invasive surgery-the robotic approach? Gland Surg. 2018; 7:1–11. PMID: 29629314.
Article13. Beger HG, Siech M, Poch B, Mayer B, Schoenberg MH. Limited surgery for benign tumours of the pancreas: a systematic review. World J Surg. 2015; 39:1557–1566. PMID: 25691214.
Article14. Kabir T, Tan ZZX, Syn N, Chung AYF, Ooi LLPJ, Goh BKP. Minimally-invasive versus open enucleation for pancreatic tumours: a propensity-score adjusted analysis. Ann Hepatobiliary Pancreat Surg. 2019; 23:258–264. PMID: 31501815.
Article15. Goh BKP, Low TY, Kam JH, Lee SY, Chan CY. Initial experience with laparoscopic and robotic surgery for the treatment of periampullary tumours: single institution experience with the first 30 consecutive cases. ANZ J Surg. 2019; 89:E137–E141. PMID: 30805992.
Article16. Bencini L, Annecchiarico M, Farsi M, Bartolini I, Mirasolo V, Guerra F, et al. Minimally invasive surgical approach to pancreatic malignancies. World J Gastrointest Oncol. 2015; 7:411–421. PMID: 26690680.
Article17. Ore AS, Barrows CE, Solis-Velasco M, Shaker J, Moser AJ. Robotic enucleation of benign pancreatic tumors. J Vis Surg. 2017; 3:151. PMID: 29302427.
Article18. Tian F, Hong XF, Wu WM, Han XL, Wang MY, Cong L, et al. Propensity score-matched analysis of robotic versus open surgical enucleation for small pancreatic neuroendocrine tumours. Br J Surg. 2016; 103:1358–1364. PMID: 27480993.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enucleation of Pancreatic Endocrine Tumor Following Pancreatic Duct Stenting: A Case Report
- Unduly extensive uncinate process of pancreas in conjunction with pancreatico-duodenal fold
- Morphometry of the Uncinate Process, Vertebral Body, and Lamina of the C3–7 Vertebrae Relevant to Cervical Spine Surgery
- Laparoscopic Enucleation of a Nonfunctioning Neuroendocrine Tumor of the Pancreas
- Surgical Results of Pancreatic Neuroendocrine Tumors